Introduction
Cognitive function tests evaluate an individualŌĆÖs memory, attention, frontal lobe/executive function, and language-related functions [
1,
2]. Therefore, they structuralize overall cognitive function and help with a subjectŌĆÖs diagnosis and anticipated prognosis [
3,
4]. Common memory function tests include sub-questions of the Mini-Mental State Examination, the verbal learning test (VLT), and the Rey Complex Figure Test (RCFT) [
5,
6]. However, they are sophisticated and time consuming, and it is difficult to perform them quickly at bedside or in an outpatient clinic. In addition, since conventional memory function tests use memory registration of predefined items, daily repetitive testing to assess improvement is difficult due to the learning curve effect. Furthermore, conventional memory tests can be biased by attention deficit, depressive mood, or pseudodementia [
7,
8].
Clinicians have used the food memory test (FMT) to evaluate patient memory function empirically, but this function has never been studied structurally. Since people attend to what they eat during a meal, the attention factor can be minimized in this test. However, FMT is uncomplicated, especially in Korea, as many people eat soup and a main dish for each meal.
We tested the FMT for reliability and usability as well as whether it could be substituted as a conventional memory test. In this study, subjects needing the memory function test in the neurology clinic were enrolled for FMT along with a correlation analysis and a conventional cognitive function test.
Methods
We prospectively performed a FMT and a standard cognitive function test set (Seoul Neuropsychological Screening Battery, SNSB) [
1,
2] in patients admitted to or visiting the neurology clinic at Seoul National University Hospital for a cognitive function evaluation. The time from onset of cognitive issues to the cognitive function test was not controlled because tests should be applicable and reliable at any time point regardless of disease phase. This study was approved by the Institutional Review Board of Seoul National University Hospital, South Korea (No. H-1912-078-1088). All participants or their legal representatives provided written informed consent.
For the FMT, we asked each patient what they ate as a soup and main dish at the last meal (FMT1) and the second-to-last meal (FMT2), a total of four questions. We collected information about the food consumed from the hospital diet data or from caregivers. If they answered the correct menu (soup and main dish) in one meal, we scored ŌĆ£pass,ŌĆØ assigning them to the FMT-pass group. If they did not answer or indicated the wrong food, we scored ŌĆ£fail,ŌĆØ assigning them to the FMT-fail group. If the patient had only one of soup or main dish, we used the single food to score pass or fail.
We performed SNSB using a standardized scoring scale [
9]. Among the items in the SNSB, some were used for a comparison analysis with the FMT; the Korean version of the Mini-Mental State Exam (K-MMSE), the Geriatric Depression Scale (GDS), the Seoul Verbal Learning Test (SVLT; immediate recall, delayed recall, and recognition), the RCFT (immediate recall and delayed recall), the contrasting program, the Go/No-go test, the fist-edge-palm, alternating hand movement, alternating square and triangle, the Luria loop, the Controlled Oral Word Association Test, spontaneous speech, comprehension, repetition, finger naming, right-left orientation, body part identification, calculation, the praxis test, and the digit span test.
Patients were categorized by diagnosis, location of brain lesion, and medications. We categorized diagnosis as autoimmune encephalitis, infectious diseases, tumors, degenerative diseases, or normal. The location of the brain lesion was divided into dominant hemisphere, temporal lobe, frontal lobe, parietal lobe, and Papez circuit (hippocampal formation, entorhinal cortex, fornix, mammillary bodies, anterior thalamus, and cingulum) [
10]. Medications were categorized into antiepileptic drugs, antipsychotics, antidepressants, and sedatives. Sedatives were defined as central nervous system (CNS) depressants, such as barbiturates and benzodiazepines. Brain lesion location and drug categories could overlap if there were multiple lesions or medications.
Data were analyzed with IBM SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and were expressed as mean ┬▒ standard deviation. The Student t-test or the Mann-Whitney U-test was used for independent data. For categorical variables, Fisher exact test (np < 5 or n [1 ŌĆō p] < 5) or Pearson chi-square test (np Ōēź 5 and n [1 ŌĆō p] Ōēź 5) was used for independent data. The independent t-test or Fisher exact test was used to compare data, and a p-value of <0.05 was considered significant. Binary logistic regression was used to identify key independent variables.
Results
Clinical characteristics of patients
We enrolled a total of 27 patients, including 15 males and 12 females, with a mean age of 50.4 ┬▒ 22.2 years (range, 18ŌĆō83 years) (
Table 1). Among them, 16 patients had autoimmune encephalitis, four had CNS infection (one intracranial tuberculoma, one herpes simplex virus (HSV)-1 encephalitis, one HSV-2 encephalitis, and one HSV encephalitis), two had tumors (two primary CNS lymphomas), four had degenerative diseases (three dementia and one hydrocephalus), and one was a normal volunteer. Of those with brain lesions, 17 had lesion in the dominant hemisphere, 14 in the temporal lobe, 10 in the frontal lobe, four in the parietal lobe, and four in the Papez circuit. Medications were also categorized; 16 used antiepileptic drugs, three used antipsychotics, three used sedatives, and one used antidepressants.
Among the 27 patients, 12 passed the FMT1 (44.4%) and nine passed the FMT2 (33.3%). When comparing the differences of clinical characteristics between the FMT1-pass and FMT1-fail groups, there was no variation in age, sex, diagnosis, or medication profiles. However, the FMT1-fail group had a higher frequency of non-frontal brain lesions (p = 0.004). When comparing clinical characteristics between the FMT2-pass and FMT2-fail groups, there was no difference in age, sex, diagnosis, medication profiles, and brain lesions (
Table 1).
FMT1 predicted the conventional memory function test score
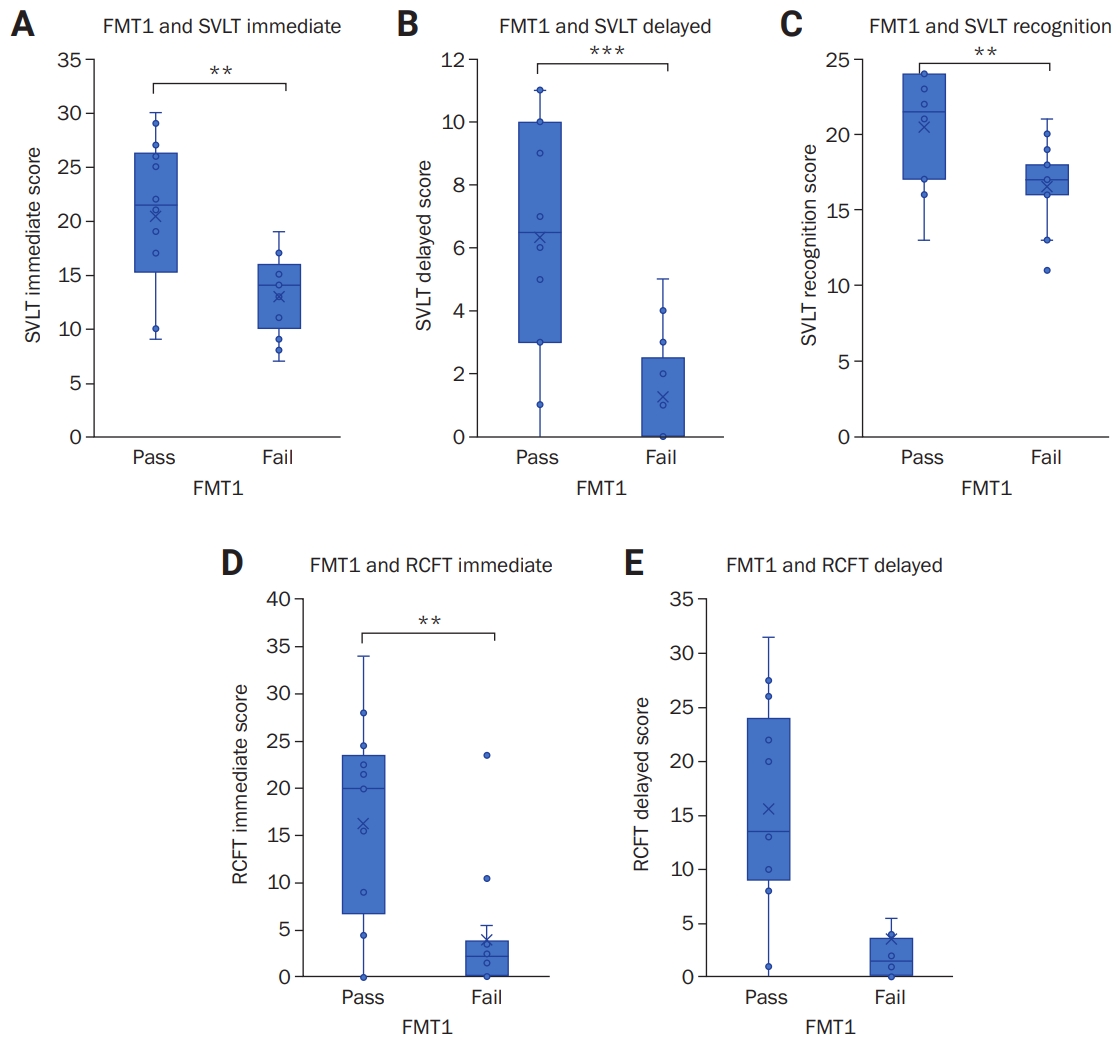
We analyzed correlation and reliability of FMT1 to the conventional cognitive function tests. Meal-to-test time (MTT) and modified Rankin scale were consistent between the FMT1/FMT2-pass group and the FMT1/FMT2-fail group. In K-MMSE subitems, the FMT1-pass group showed higher scores compared with the FMT1-fail group in time-place orientation (FMT1-pass, 9.2 ┬▒ 2.0 and FMT1-fail, 6.5 ┬▒ 3.4; p = 0.016) with three-word recall tests (2.6 ┬▒ 0.9 and 1.5 ┬▒ 1.3, p = 0.015). In SVLT, the FMT1-pass group showed higher scores than the FMT1-fail group in immediate recall (FMT1-pass, 20.4 ┬▒ 7.5 and FMT1-fail, 13.0 ┬▒ 3.8; p = 0.007), delayed recall (6.3 ┬▒ 3.9 and 1.3 ┬▒ 1.7, p = 0.001), and recognition (20.5 ┬▒ 3.8 and 16.6 ┬▒ 2.8, p = 0.006) (
Figure 1AŌĆō
C). In the RCFT, the FMT1-pass group showed higher scores than the FMT1-fail group in immediate recall (FMT1-pass, 16.3 ┬▒ 11.5 and FMT1-fail, 4.0 ┬▒ 6.3; p = 0.006) and delayed recall (15.7 ┬▒ 10.6 and 3.5 ┬▒ 6.0, p = 0.004) (
Figure 1D,
E). No tests evaluated attention, frontal, or executive function; however, language and related function showed a significant difference between the FMT1-pass and FMT1-fail groups, except in right-left orientation. GDS showed no significant difference between the FMT1-pass group and FMT1-fail group.
We also analyzed the correlation and reliability of FMT2 to the conventional cognitive function tests. The FMT2-pass group showed a significant difference from the FMT2-fail group, but only in time-place orientation, three-word recall, SVLT immediate recall, SVLT delayed recall, and the contrasting program (
Table 2).
We further determined the key domains in the conventional cognitive function test, predicting FMT1 performance using logistic regression analysis. While other SNSB categories showed a nonsignificant association with FMT1 results, SVLT delayed recall score was significant (p = 0.010), and the odds ratio was 1.745, suggesting that the FMT1 test reflects recent memory function.
Discussion
We showed that FMT1 is a reliable memory test that can predict the results of conventional memory tests. FMT1 was not influenced by attention, frontal lobe function tests, or depression. This indicates that FMT can be conducted in environments where patients find it difficult to focus on the test itself, such as the emergency room, and in patients with attention deficit due to depression including those with pseudodementia. FMT1 was not affected by MTT, so it can be performed at any time within a range of 1 to 6 hours after the last meal. FMT1-fail patients had more non-frontal lesions, which could be because FMT1-fail patients had greater involvement of memory-related brain structures outside of the frontal lobe. In the same context, the FMT1-fail group showed more frequent failure in the right-left orientation test, which is localized to the left parietal lobe [
11]. FMT1 was more reliable than FMT2, at least in correlation with conventional cognitive function tests.
FMT1 is more likely to represent the recent verbal and episodic memory. This is supported by the finding that SVLT delayed recall predicts FMT1 performance. The delayed recall of the VLT is a sensitive measure for diagnosis of mild amnestic cognitive impairment and early Alzheimer disease [
12]. While language functions were not different in the FMT1-pass and fail groups in the current study, patients who had naming difficulties or loss of semantic knowledge about food could not perform the test. Furthermore, patients with language problems could also not perform the conventional memory function test, while some patients with hand weakness could not perform the RCFT, as experienced in our testing (
Table 2).
We also showed that FMT1 is a reliable test that predicts conventional memory tests. Since the number of patients and number of disease categories are limited, we must collect more data and conduct this test in a larger population. Since our patients were mainly enrolled in the autoimmune neurology clinic, the test must be reproduced in various disease categories. In addition, because food memory can be biased by variability and novelty of meals, the viability of this test with other ethnicities, or with different food cultures, must be validated separately. Moreover, hospital meals for admitted patients have limited variability. This study could not confirm intra- and inter-rater reliability due to difficulties in repeating the FMT on the same patient. Still, we believe that the FMT is useful for daily clinical practice in neurology.
In conclusion, FMT1-pass or fail reflected key memory function tests, similar to the delayed recall of the VLT. Furthermore, the FMT1 score was not biased by depression (pseudodementia) or attention deficit, rendering it more reliable. Because the FMT1 takes only several seconds to complete, we suggest it as a quick, simple, bedside-compatible memory test that warrants further studies.
Acknowledgments
This study was supported by Student Research Program of Seoul National University College of Medicine (2019).
Figure┬Ā1.
Correlation between FMT1 and conventional memory tests
Correlation with the immediate score (A), delayed score (B), and recognition score (C) of Seoul Verbal Learning Test (SVLT); correlation with the immediate score (D) and delayed score (E) of Rey Complex Figure Test (RCFT). Whiskers indicate the range of scores. ***p < 0.001, **p < 0.01.
FMT1, food memory test for the last meal.
Table┬Ā1
Demographic and clinical characteristics of patients
|
Characteristic |
Total |
FMT1
|
FMT2
|
|
Pass |
Fail |
p-value |
Pass |
Fail |
p-value |
|
No. of patients |
27 |
12 |
15 |
|
9 |
18 |
|
|
Age (yr) |
50.4 ┬▒ 22.2 |
45.1 ┬▒ 24.0 |
54.7 ┬▒ 21.4 |
0.280 |
44.7 ┬▒ 24.3 |
53.3 ┬▒ 22.0 |
0.359 |
|
Male sex |
15 (55.6) |
7 (58.3) |
8 (53.3) |
0.795 |
5 (55.6) |
10 (55.6) |
>0.999 |
|
Diagnosisa)
|
|
|
|
|
|
|
ŌĆāAutoimmune encephalitis |
16 (59.3) |
7 (58.3) |
9 (60.0) |
0.930 |
7 (77.8) |
9 (50.0) |
0.231 |
|
ŌĆāCNS infection |
4 (14.8) |
2 (16.7) |
2 (13.3) |
>0.999 |
1 (11.1) |
3 (16.7) |
>0.999 |
|
ŌĆāTumor |
2 (7.4) |
1 (8.3) |
1 (6.7) |
>0.999 |
0 (0) |
2 (11.1) |
0.538 |
|
ŌĆāDegenerative disease |
4 (14.8) |
1 (8.3) |
3 (20.0) |
0.605 |
0 (0) |
4 (22.2) |
0.268 |
|
ŌĆāNormal |
1 (3.7) |
1 (8.3) |
0 (0) |
0.444 |
1 (11.1) |
0 (0) |
0.333 |
|
MRI lesion |
|
|
|
|
|
|
ŌĆāDominant hemisphereb)
|
17 (63.0) |
7 (58.3) |
10 (66.7) |
0.656 |
6 (66.7) |
11 (61.1) |
0.778 |
|
ŌĆāTemporal lobe |
14 (51.9) |
5 (41.7) |
9 (60.0) |
0.343 |
3 (33.3) |
11 (61.1) |
0.173 |
|
ŌĆāFrontal lobe |
10 (37.0) |
8 (66.7) |
2 (13.3) |
0.004 |
5 (55.6) |
5 (27.8) |
0.219 |
|
ŌĆāParietal lobe |
4 (14.8) |
3 (25.0) |
1 (6.7) |
0.294 |
3 (33.3) |
1 (5.6) |
0.093 |
|
ŌĆāPapez circuit |
4 (14.8) |
2 (16.7) |
2 (13.3) |
>0.999 |
2 (22.2) |
2 (11.1) |
0.582 |
|
Drug |
|
|
|
|
|
|
ŌĆāAntiepileptic drugs |
16 (59.3) |
6 (50.0) |
10 (66.7) |
0.381 |
6 (66.7) |
10 (55.6) |
0.580 |
|
ŌĆāAntipsychotics |
3 (11.1) |
0 (0) |
3 (20.0) |
0.231 |
2 (22.2) |
1 (5.6) |
0.250 |
|
ŌĆāAntidepressants |
1 (3.7) |
0 (0) |
1 (6.7) |
>0.999 |
0 (0) |
1 (5.6) |
>0.999 |
|
ŌĆāSedatives |
3 (11.1) |
0 (0) |
3 (20.0) |
0.231 |
1 (11.1) |
2 (11.1) |
>0.999 |
Table┬Ā2
Food memory test and patient Seoul Neuropsychological Screening Battery Scores
|
Characteristic |
Total |
FMT1
|
FMT2
|
|
Pass |
Fail |
p-value |
Pass |
Fail |
p-value |
|
No. of patients |
27 |
12 |
15 |
|
9 |
18 |
|
|
MTT (hr) |
|
|
|
|
|
|
|
|
ŌĆāFirst |
2.8 ┬▒ 1.2 |
2.6 ┬▒ 1.6 |
2.9 ┬▒ 0.9 |
0.609 |
2.2 ┬▒ 1.3 |
3.1 ┬▒ 1.1 |
0.080 |
|
ŌĆāSecond |
12.1 ┬▒ 5.2 |
14.2 ┬▒ 4.3 |
10.1 ┬▒ 5.4 |
0.045 |
12.7 ┬▒ 5.0 |
11.7 ┬▒ 5.5 |
0.674 |
|
ŌĆāmRS |
3 (0ŌĆō4) |
2.5 (0ŌĆō4) |
3 (2ŌĆō4) |
0.110 |
3 (0ŌĆō4) |
3 (2ŌĆō4) |
0.891 |
|
K-MMSE |
|
|
ŌĆāTime-place orientation |
7.7 ┬▒ 3.1 |
9.2 ┬▒ 2.0 |
6.5 ┬▒ 3.4 |
0.016 |
9.6 ┬▒ 0.5 |
6.7 ┬▒ 3.4 |
0.003 |
|
ŌĆāThree-word registration |
2.9 ┬▒ 0.3 |
2.9 ┬▒ 0.3 |
2.9 ┬▒ 0.4 |
0.695 |
3.0 ┬▒ 0.0 |
2.8 ┬▒ 0.4 |
0.083 |
|
ŌĆāThree-word recall |
2.0 ┬▒ 1.3 |
2.6 ┬▒ 0.9 |
1.5 ┬▒ 1.3 |
0.015 |
2.7 ┬▒ 0.7 |
1.6 ┬▒ 1.4 |
0.013 |
|
ŌĆāLanguage |
6.9 ┬▒ 1.6 |
7.3 ┬▒ 0.9 |
6.5 ┬▒ 1.9 |
0.189 |
7.0 ┬▒ 1.2 |
6.8 ┬▒ 1.7 |
0.798 |
|
ŌĆāTotal |
23.1 ┬▒ 6.2 |
25.4 ┬▒ 4.6 |
21.3 ┬▒ 6.7 |
0.081 |
25.4 ┬▒ 3.6 |
21.9 ┬▒ 6.9 |
0.095 |
|
SVLTa)
|
|
|
ŌĆāImmediate recall |
16.3 ┬▒ 6.8 |
20.4 ┬▒ 7.5 |
13.0 ┬▒ 3.8 |
0.007 |
20.3 ┬▒ 7.8 |
14.3 ┬▒ 5.3 |
0.026 |
|
ŌĆāDelayed recall |
3.5 ┬▒ 3.8 |
6.3 ┬▒ 3.9 |
1.3 ┬▒ 1.7 |
0.001 |
6.1 ┬▒ 4.0 |
2.2 ┬▒ 3.1 |
0.010 |
|
ŌĆāRecognition |
18.4 ┬▒ 3.8 |
20.5 ┬▒ 3.8 |
16.6 ┬▒ 2.8 |
0.006 |
19.8 ┬▒ 4.0 |
17.7 ┬▒ 3.6 |
0.177 |
|
RCFTb)
|
|
|
ŌĆāImmediate recall |
9.4 ┬▒ 10.8 |
16.3 ┬▒ 11.5 |
4.0 ┬▒ 6.3 |
0.006 |
15.0 ┬▒ 10.7 |
6.8 ┬▒ 10.0 |
0.074 |
|
ŌĆāDelayed recall |
8.9 ┬▒ 10.2 |
15.7 ┬▒ 10.6 |
3.5 ┬▒ 6.0 |
0.004 |
14.0 ┬▒ 10.3 |
6.5 ┬▒ 9.5 |
0.085 |
|
Attention |
|
|
ŌĆāDigit span: forward |
5.3 ┬▒ 2.1 |
6.0 ┬▒ 1.9 |
4.7 ┬▒ 2.2 |
0.292 |
5.6 ┬▒ 2.2 |
5.2 ┬▒ 2.1 |
0.835 |
|
ŌĆāDigit span: backward |
3.6 ┬▒ 1.6 |
2.9 ┬▒ 1.3 |
2.9 ┬▒ 1.3 |
0.256 |
4.0 ┬▒ 1.7 |
3.3 ┬▒ 1.6 |
0.466 |
|
Language and related functions |
|
|
ŌĆāComprehension |
20 (74.1) |
11 (91.7) |
9 (60.0) |
0.062 |
8 (88.9) |
12 (66.7) |
0.214 |
|
ŌĆāRepetition |
13.7 ┬▒ 1.9 |
14.2 ┬▒ 0.9 |
13.4 ┬▒ 2.4 |
0.256 |
14.3 ┬▒ 1.1 |
13.4 ┬▒ 2.1 |
0.239 |
|
ŌĆāFinger naming |
24 (88.9) |
11 (91.7) |
13 (86.7) |
0.681 |
9 (100) |
15 (83.3) |
0.194 |
|
ŌĆāRight-left orientation |
20 (74.1) |
12 (100) |
8 (53.3) |
0.008 |
9 (100) |
11 (61.1) |
0.059 |
|
ŌĆāBody part identification |
20 (74.1) |
10 (83.3) |
10 (66.7) |
0.326 |
7 (77.8) |
13 (72.2) |
0.756 |
|
ŌĆāCalculation |
8.8 ┬▒ 3.8 |
9.3 ┬▒ 3.7 |
8.4 ┬▒ 4.0 |
0.540 |
9.4 ┬▒ 3.4 |
8.5 ┬▒ 4.9 |
0.557 |
|
ŌĆāPraxis |
26 (96.3) |
11 (91.7) |
15 (100) |
0.255 |
9 (100) |
17 (94.4) |
0.471 |
|
ŌĆāIdeomotor |
4.1 ┬▒ 1.2 |
4.6 ┬▒ 0.9 |
3.7 ┬▒ 1.2 |
0.148 |
4.4 ┬▒ 1.0 |
3.9 ┬▒ 1.2 |
0.578 |
|
Frontal/executive function |
|
|
ŌĆāContrasting program |
16.9 ┬▒ 6.6 |
18.3 ┬▒ 5.8 |
15.7 ┬▒ 7.1 |
0.317 |
19.7 ┬▒ 0.7 |
15.5 ┬▒ 7.7 |
0.036 |
|
ŌĆāGo/No-go test |
14.8 ┬▒ 7.9 |
16.8 ┬▒ 6.8 |
13.1 ┬▒ 8.6 |
0.233 |
15.9 ┬▒ 7.4 |
14.2 ┬▒ 8.3 |
0.614 |
|
ŌĆāFist-edge-palm |
21 (77.8) |
10 (83.3) |
11 (73.3) |
0.535 |
8 (88.9) |
13 (72.2) |
0.326 |
|
ŌĆāAlternating hand movement |
22 (81.5) |
10 (83.3) |
12 (80.0) |
0.446 |
8 (88.9) |
14 (77.8) |
0.147 |
|
ŌĆāAlternating square and triangle |
17 (63.0) |
7 (58.3) |
10 (66.7) |
0.678 |
6 (66.7) |
11 (61.1) |
0.607 |
|
ŌĆāLuria loop |
18 (66.7) |
8 (66.7) |
10 (66.7) |
0.943 |
6 (66.7) |
12 (66.7) |
0.819 |
|
COWATc)
|
|
|
ŌĆāAnimal |
10.7 ┬▒ 5.4 |
12.6 ┬▒ 6.2 |
9.2 ┬▒ 4.7 |
0.109 |
12.2 ┬▒ 5.5 |
9.9 ┬▒ 5.4 |
0.313 |
|
ŌĆāSupermarket |
9.2 ┬▒ 5.0 |
11.4 ┬▒ 4.6 |
7.4 ┬▒ 4.6 |
0.030 |
10.3 ┬▒ 4.9 |
8.6 ┬▒ 5.0 |
0.405 |
|
ŌĆāPhonemic sum |
19.4 ┬▒ 13.4 |
23.7 ┬▒ 14.4 |
15.6 ┬▒ 11.8 |
0.132 |
23.4 ┬▒ 15.6 |
17.2 ┬▒ 11.8 |
0.267 |
|
GDS |
12.9 ┬▒ 8.1 |
13.1 ┬▒ 8.2 |
12.7 ┬▒ 8.4 |
0.898 |
10.3 ┬▒ 8.4 |
14.1 ┬▒ 7.9 |
0.262 |
References
1. Kang Y, Na DL. Seoul Neuropsychological Screening Battery. Incheon: Human Brain Research & Consulting Co.; 2003.
2. Kang Y, Jahng S, Na DL. Seoul Neuropsychological Screening Battery, 2nd ed (SNSB-II). Seoul: Human Brain Research & Consulting Co.; 2012.
3. Folstein MF, Folstein SE, McHugh PR. ŌĆ£Mini-mental stateŌĆØ. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189ŌĆō198.


4. Jansen WJ, Handels RL, Visser PJ, et al. The diagnostic and prognostic value of neuropsychological assessment in memory clinic patients. J Alzheimers Dis 2017;55:679ŌĆō689.


7. Everaert J, Duyck W, Koster EH. Attention, interpretation, and memory biases in subclinical depression: a proof-of-principle test of the combined cognitive biases hypothesis. Emotion 2014;14:331ŌĆō340.


8. Small GW, Liston EH, Jarvik LF. Diagnosis and treatment of dementia in the aged. West J Med 1981;135:469ŌĆō481.


10. Shah A, Jhawar SS, Goel A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J Clin Neurosci 2012;19:289ŌĆō298.










 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print