Respiratory virus-related meningoencephalitis in adults
Article information
Abstract
Purpose
Respiratory viruses (RVs) are pathogens that can cause central nervous system (CNS) infection, but previous research has been limited to a pediatric population. In recent years, several cases of adult RV meningoencephalitis have begun to be reported. We decided to research the CNS infection of RV in the entire neuroinfection registry.
Methods
We retrospectively reviewed the neurologic infection registry of Seoul National University (Seoul, Korea). Among a total of 661 patients in the registry, 10 adult patients were diagnosed with RV-related meningoencephalitis on RV multiplex polymerase chain reaction (PCR) screening test. We analyzed the clinical presentation, laboratory findings, and clinical course of the 10 patients.
Results
Three patients were definite RV meningoencephalitis who had positive PCR results from cerebrospinal fluid. The other seven patients were diagnosed with probable RV meningoencephalitis if they had positive PCR results in the sputum and negative results in other extensive workup.
Conclusion
RV-related meningoencephalitis should be considered a possible etiology in adult meningoencephalitis patients. To diagnose these viruses, screening test of RV PCR is recommended even in patients without upper respiratory infection symptoms.
Introduction
The respiratory virus (RV) group consists of viruses such as adenovirus; respiratory syncytial virus (RSV) A and B; rhinovirus A and B; coronavirus, influenza A and B; parainfluenza 1, 2, 3; and metapneumovirus. RVs mainly cause upper or lower respiratory tract infections, but they can also cause central nervous system (CNS) infection, mostly in children [1]. Adult cases of RV-related meningoencephalitis have been reported in a limited number of patients, predominantly those with influenza virus [2]. However, up to 66% of adult encephalitis cases fail to have a causative pathogen identified despite extensive diagnostic laboratory tests [3]. Therefore, further research is needed to identify unusual but detectable pathogens such as RV. We retrospectively reviewed the neurological infection registry, and analyzed the pathogenic etiology of the CNS infections, including the RVs.
Methods
From March 2012 to December 2015, patients visiting Seoul National University Hospital (Seoul, Korea) with a clinical suspicion of CNS infection were enrolled in the neurological infection registry of Seoul National University Hospital (Seoul, Korea). The inclusion criteria were as follows; (1) clinical suspicion of CNS infection, (2) ≥18 years of age, and (3) cerebrospinal fluid (CSF) pleocytosis. The exclusion criteria were as follows; (1) CNS infection related to a recent surgical intervention, or (2) the presence of an additional etiology, such as autoimmune encephalitis or metabolic encephalopathy.
All patients in this registry underwent RV multiplex polymerase chain reaction (PCR) (Anyplex II RV 16 Detection; Seegene Inc., Seoul, Korea) analysis of CSF and sputum. This test provides qualitative analysis for 16 species of RV (adenovirus, influenza A and B, parainfluenza virus 1/2/3/4, rhinovirus A/B/C, RSV A and B, bocavirus 1/2/3/4, metapneumovirus, coronavirus 229E, coronavirus NL63, coronavirus OC43, and enterovirus). Other extensive diagnostic tests were performed, which included CSF culture, CSF PCR, and serum antibody tests for bacteria and viruses. Work-ups for rare pathogens such as tuberculosis, fungus, and parasites were also performed. A clinical diagnosis of meningitis or encephalitis was made based on the initial manifestation of symptoms. Meningitis was characterized by fever and headache without any neurological symptoms. Encephalitis was characterized by headache, altered level of consciousness, and symptoms and signs of cerebral dysfunction such as cognitive impairment, behavioral changes, focal neurologic abnormalities, and seizures [4]. Individual patients were treated according to clinical decisions made by an expert physician from the neurological infection department. Because of its retrospective nature, this study was exempted from the approval of the Institutional Review Board and the written informed consent of the subject.
Results
A total of 661 patients were diagnosed with CNS infection and enrolled in the registry. Among these 661 CNS infection patients, 351 had meningitis, 275 encephalitis, and 35 brain abscess. Etiological pathogens were confirmed in 421 patients (63.7%). Viral infection (253, 60.1%) was the major etiology in pathogen-confirmed CNS infection, followed by bacterial infection (105, 24.9%), mycobacterial infection (25, 5.9%) and fungal infection (21, 5.0%). In pathogen-confirmed viral infection, EBV was the leading etiology (73, 28.9%) followed by herpes simplex virus (HSV; 62, 24.5%), varicella-zoster virus (VZV; 53, 20.9%), and enterovirus (51, 20.2%).
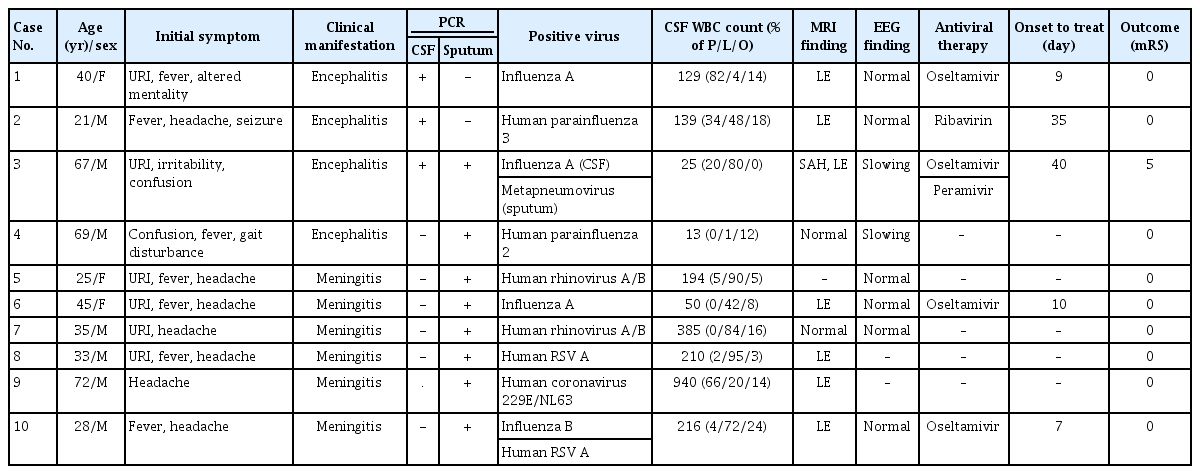
A total of 10 patients were diagnosed with RV-related meningoencephalitis. RV PCR of CSF or sputum yielded positive results, with no other findings on extensive workup. The median age of the patients was 38 years (range, 21–72 years). Four patients were clinically diagnosed with encephalitis and the others were diagnosed with meningitis. Six patients (60.0%) had prior upper respiratory infection (URI) symptoms before manifestation of CNS infection. Leptomeningeal enhancement was the most frequent finding (70.0%) observed on brain magnetic resonance imaging (MRI). Antiviral therapy was administered in 3 of 4 encephalitis patients and 2 of 7 meningitis patients. All of the patients recovered fully without any neurological sequelae, except for one patient (patient 3) who deteriorated despite antiviral treatment (Table 1).
We classified these patients into two different groups according to PCR results. The first was the “definite” group, in which RV presence was confirmed by CSF PCR, and the second was the “probable” group, in which RV presence could be confirmed only by sputum PCR.
Definite respiratory virus meningoencephalitis
Three patients were classified into the “definite” group. Two patients had positive PCR results for influenza A in CSF. The other patient had positive results for human parainfluenza 3 virus in CSF.
Patient 1 was a 40-year-old female. She visited the emergency room for altered mentality and fever lasting 5 days. Before the onset of neurologic symptoms, she complained of cough, rhinorrhea, and mild fever. Initial CSF revealed pleocytosis with a white blood cell (WBC) count of 129/mm3 (polymorphonucleocytes, 82%; lymphocytes, 4%; other cells, 14%), and elevated protein (153 mg/dL). Initial brain MRI showed leptomeningeal enhancement, but electroencephalography (EEG) did not demonstrate abnormal findings. On extensive diagnostic workup, CSF PCR was positive for influenza A virus. She was treated with oseltamivir and recovered fully after 2 weeks of antiviral therapy; she was discharged at a modified Rankin Scale (mRS) score of 0.
Patient 2 was a 21-year-old male who presented with first-onset seizure. He had a generalized tonic-clonic seizure after complaining of headache for 5 days. Initial CSF showed pleocytosis with red blood cells (RBC) of 12/mm3, a WBC of 139/mm3 (polymorphonucleocytes, 34%; lymphocytes, 48%; other cells, 18%), and elevated protein (52 mg/dL). RV PCR of CSF was positive for human parainfluenza virus 3; otherwise, there were no abnormal findings on extensive diagnostic workup. Brain MRI showed leptomeningeal enhancement and EEG demonstrated no focal epileptiform discharge but did reveal generalized intermittent rhythmic delta activity. He was treated with ribavirin for 1 week, after which he was clinically recovered and did not complain of any headache or seizure. Follow-up EEG was also normal after ribavirin treatment.
Patient 3 was a 67-year-old male who had been hospitalized previously for headache, right-side weakness, and transcortical motor aphasia. Prior to headache, he suffered from cough and rhinorrhea for 1 month. Brain computed tomography at a previous hospital showed cortical subarachnoid hemorrhage (SAH). He was treated with corticosteroids in order to reduce intracranial pressure. Initial CSF results revealed pleocytosis with RBC of 270/mm3, WBC of 25/mm3 (lymphocytes, 80%; polymorphonucleocytes, 20%), and elevated protein (214.7 mg/dL). He recovered partially and was discharged after 1 week of steroid treatment. However, before long, he started to show confused mentality again and was transferred to our hospital for a second opinion. Follow-up CSF analysis in our hospital revealed persistent pleocytosis with an RBC of 18/mm3 and WBC of 16/mm3 (lymphocytes, 13; other cells, 3), and elevated protein (137 mg/dL). On RV PCR, the CSF specimen was positive for influenza A and the sputum specimen was positive for human metapneumovirus. On brain MRI, cortical SAH (Figure 1A) was aggravated and leptomeningeal enhancement (Figure 1B) was observed. Initially, acyclovir was administered for 1 week but was changed to oseltamivir after CSF PCR results were obtained. Although we treated him with oseltamivir for 2 weeks and follow-up CSF PCR results were negative, his mental status worsened. Finally, we administered intravenous peramivir, but the patient entered a persistent vegetative state (mRS score 5) without clinical response.
Probable respiratory virus meningoencephalitis
We classified the other seven patients into the clinically “probable” group. Patients in this group were clinically diagnosed with meningoencephalitis and no pathogens were found on extensive workup other than positive RV PCR of sputum. There were two human rhinovirus A/B infections, one of influenza A, one of human parainfluenza virus 2, one human coronavirus 229E/NL63, and one case with both influenza B and human RSV A. Four of these patients had URI symptoms prior to headache. Two patients whose PCR results were positive for influenza A were treated with oseltamivir, and the other patients underwent conservative management. Four patients exhibited leptomeningeal enhancement on MRI. Specific clinical information is presented in Table 1.
Discussion
Here, we demonstrated that, although rare, RVs can be a causative pathogen of meningoencephalitis in adults. According to our data from an adult population, 1.5% of total CNS infection patients were diagnosed with RV-related meningoencephalitis. This accounts for 4.0% of the total CNS viral infections.
Recently, neurologic manifestations of respiratory viral infection have come to the fore. RVs can invade the CNS through either a hematogenous route or a peripheral nerve route [5]. Many cases of RV-associated meningoencephalitis have been reported in adult patients with adenovirus [6], bocavirus [7], influenza A [8-10], influenza B [11,12], parainfluenza [13], and metapneumovirus [14-17]. Reports of meningoencephalitis caused by diverse types of RVs are more common in pediatric cases. RV-related encephalitis comprises approximately 20% of all encephalitis cases in children aged 1 month to 15 years [2]. However, even in adults, RVs remain an important pathogenic cause of meningoencephalitis around the world.
The diagnostic strategy for RV-related meningoencephalitis is similar to other infectious meningoencephalitis. PCR and real time-PCR assays of CSF for the detection of viruses are the most reliable diagnostic tools. A wide range of PCR tests should be carried out, including RV panel, HSV-1, HSV-2, VZV, CMV, and enterovirus, among others. Serologic tests, including serum and CSF specimens, are also helpful to specific etiological diagnosis. In the case of respiratory viral infection, we believe PCR testing of sputum will help establish a diagnosis [18].
Detailed history taking about prodromal symptoms, recent travel, geographic location, exposure history, and occupation provides important clinical clues regarding infectious pathogens. Nevertheless, clinical symptoms and neurologic examination findings are similar among CNS infections. In our study, it is remarkable that a considerable portion of RV-related meningoencephalitis patients (4 of 10) did not have URI symptoms. In 2009, a prospective longitudinal study showed that respiratory pathogens are frequently detected in samples not only from children with respiratory symptoms (56%) but also from those without respiratory symptoms (40%) [19]. Therefore, we believe that a respiratory PCR panel should be routinely conducted in patients with suspected CNS infections.
Most RV-related meningoencephalitis patients showed favorable outcomes. RV-related encephalitis is treatable with properly timed antiviral therapy. In particular, influenza A encephalitis can be treated with antiviral therapy [20,21]. Influenza A encephalitis can be fatal not only in children but also in adults [22-24]. Cortical SAH can develop from influenza A infection [25,26]. In our cases, patient 3 showed a poor clinical outcome despite antiviral treatment. He already had a cortical SAH long before antiviral treatment began. The delay between onset and administration of the antiviral agent could be the reason why he was the only patient with clinical deterioration. This case showed the importance of fast and accurate diagnosis of RV-related meningoencephalitis [27]. Ribavirin, a broad-spectrum antiviral agent, is used to treat Paramyxoviridae pneumonia, such as human RSV, parainfluenza virus, and metapneumovirus [28]. Intravenous ribavirin was effective in Nipha-virus encephalitis [29], which is caused by another Paramyxoviridae disease. In our study, patient 2 was also successfully treated with ribavirin. Ribavirin is the treatment of choice when any patient is infected with RVs like those described above.
Our study has some limitations in the certainty of diagnosis. Because of the low detectability of viral nucleic acids in CSF, many cases of encephalitis were diagnosed based on the results of serological tests, antigen detection, viral culture, and nucleic acid detection from sputum, stool, urine, or blood [4]. Positive PCR results from sputum also can be indirect evidence of a diagnosis. Previous studies of RV-related meningoencephalitis and other studies of CNS infection etiology have used these indirect methods for specific etiological diagnosis [3]. Nevertheless, these indirect tools provide a lower level of confidence than PCR results from CSF and have the possibility of detecting asymptomatic coinfections limited to the upper respiratory system. This is an unavoidable limitation of our study. Therefore, we chose to divide the patients into two groups based on the certainty of diagnosis.
In conclusion, this is the first etiological study of adult RV-related meningoencephalitis in a large CNS infection registry. Clinicians should keep in mind that, although rare, RVs can cause acute meningoencephalitis in adult patients, even in those without URI symptoms.
Notes
Conflicts of Interest
Jangsup Moon, Soon-Tae Lee, Kyung-Il Park, Keun-Hwa Jung, Sang Kun Lee, Kon Chu have been editorial board of encephalitis since October 2020. They were not involved in the review process of this original article. No other potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: ST Lee, K Chu; Data curation: SJ Ahn; Formal analysis: SJ Ahn, J Moon; Funding acquisition: M Kim; Methodology: SJ Ahn, K Chu; Visualization: SJ Ahn, JS Jun; Supervision: J Sunwoo, ST Lee, KI Park, KH Jung, KY Jung, M Kim, SK Lee, K Chu; Validation: KI Park, KH Jung, KY Jung, M Kim, SK Lee; Writing–original draft: SJ Ahn, J Moon; Writing–review & editing: SJ Ahn, J Moon, J Sunwoo, JS Jun, K Chu.
Acknowledgements
This work was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2016M33C7A1914002).