|
 |
| Encephalitis > Volume 1(4); 2021 > Article |
|
Abstract
Notes
Figure 1.
Hyperintensity in the subcortical white matter was seen in the fluid-attenuated inversion recovery sequence of the brain magnetic resonance imaging.
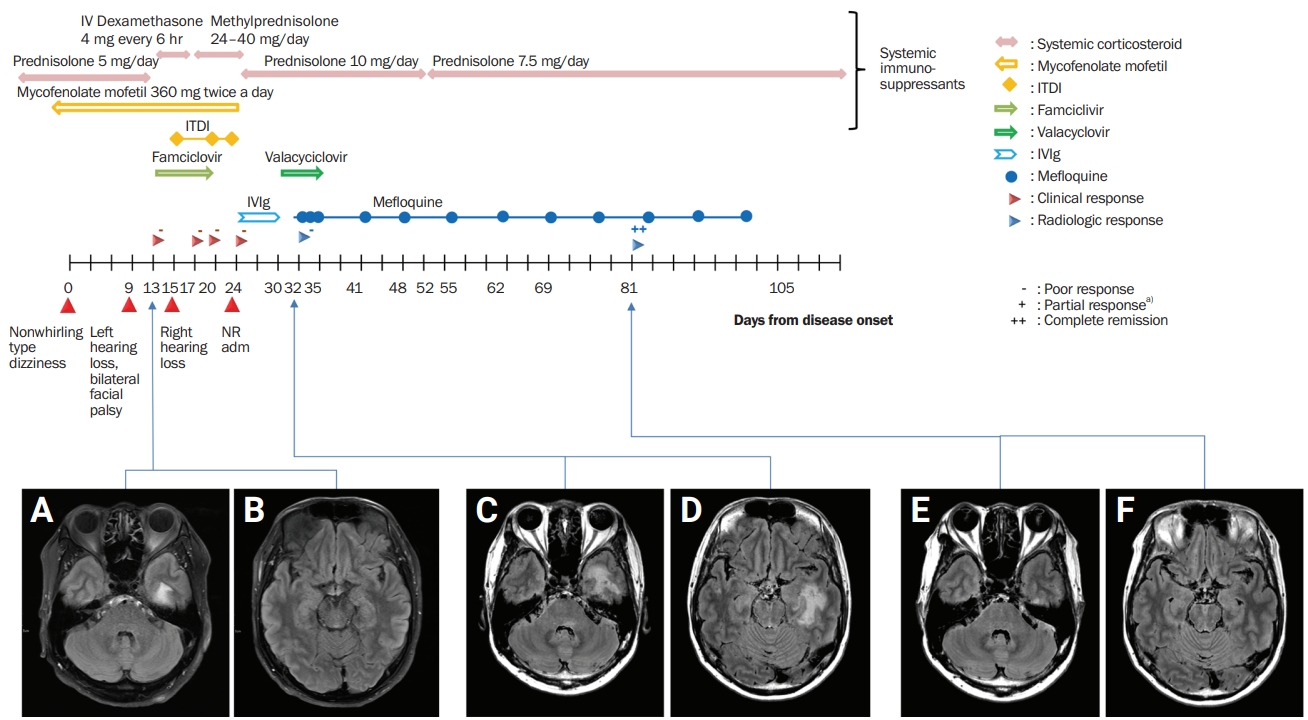
Table 1
Table 2
| Study | Age (yr)/sex | Presenting symptom | Comorbidity | Treatment | MRI finding | CSF study | Brain biopsy |
|---|---|---|---|---|---|---|---|
| Kuhle et al., 2011 [26] | 48/F | L side hypesthesia and dysesthesia | RRMS | Prednisolone | Compatible with MS | Negative PCR for JCV | Polyomavirus particles on EM |
| Nonenhancing and faintly enhancing ribbon-like lesion | |||||||
| Silverio et al., 2015 [22] | 69/M | Progressive dysarthria and R hemiparesis | Follicular lymphoma | Chemotherapy | Multiple confluent foci of FLAIR hyperintensity involving the inferior R and L frontal lobes, as well as periventricular regions | Negative PCR for JCV | Chromatin margination and viropathic change within oligodendrocytes |
| Babi et al., 2015 [27] | 75/F | Progressive L hemiplegia and global decline | Rheumatoid arthritis | Methotrexate, adalimumab | Asymmetric subcortical FLAIR HIS involving R frontoparietal subcortical WM | Negative PCR for JCV | Viral inclusion in enlarged oligodendroglial nucleus |
| Lee et al., 2019 [28] | 44/M | Dysphagia, memory disturbance, Seizure | AIDS | HAART | Multifocal patchy lesions involving subcortical region of both frontal, R temporoparietal, L thalamus, striatocapsular regions | Negative PCR for JCV | Large infected oligodendrocytes with inclusion-bearing dark nuclei |
| High JCV DNA titer of brain biopsy specimen | |||||||
| van der Kolk et al., 2016 [29] | 49/M | Aphasia, dyscalculia, hyperesthesia of the R arm, and headache | NA | NA | Large confluating asymmetric white matter hyperintensities lesions in the frontal and parietal lobes | Negative PCR for JCV | Positive PCR for JCV on the biopsy material |
| Kharfan-Dabaja et al., 2007 [30] | 51/M | Confusion and disorientation, dysnomia and progressive R upper extremity weakness → seizure → receptive aphasia, R hemiparesis, and cortical blindness | Follicular NHL and secondary myelodysplasia | GVHD prophylaxis with methotrexate , tacrolimus, alloHCT | T2 hyperintensity in periventricular white matter clustered within the L centrum semiovale | Negative PCR for JCV | Extensive demyelination, presence of naked axons, reactive gliosis, and lipid-laden macrophages |
| Occasional nuclei with a basophilic ground glass appearance, suggestive of inclusions | |||||||
| The presence of viral particles typical of the papovavirus group in multiple cells in EM | |||||||
| Chowdhary and Chamberlain 2008 [31] | 51/M | Progressive confusion, dysarthria, and visual disturbance | Myelodysplasia and NHL | Allogenic bone marrow transplantation | Multifocal T2HSI lesions including L frontal, parietal, and occipital lobes | Negative PCR for JCV for twice | Multiple enlarged, basophilic nuclei of infected oligodendrocytes intranuclear accumulation of spherical and filamentous viral particles typical of the papovavirus group |
| Vidarsson et al., 2002 [32] | 63/M | Progressive memory loss and R visual disturbances | Follicular lymphoma | Fludarabine, mitoxantrone, dexamethasone | Multifocal T2HSI lesion in L parieto-occipital area | Negative PCR for JCV | Abnormal astrocytes with hyperchromaticnuclei, and oligodendrocytes with enlarged nuclei and “ground glass”' appearance |
| Landry et al. 2008 [33] | 31/F | L facial palsy and L sided weakness | Job’s syndrome (HIES) | IVIg | Atypical T2HSI pattern | Negative PCR for JCV | Demyelination, myelin-debris-laden foamy macrophages, enlarged nuclei but no definitive intranuclear inclusions in oligodendroglial cells and no bizarre astrocytes |
| Multiple sclerosis treated with high-dose corticosteroid and plasma exchange | Polyomavirus particles in EM finding | ||||||
| Sikkema et al., 2013 [34] | 74/F | Progressive symptoms of motor imbalance, fatigue, weight loss, and impaired cognitive function | DLBCL | RCHOP | T2HSI lesions in L thalamus/mesencephalon, R subcortical frontal lobe | Negative PCR for JCV | Reactive gliosis and in the middle of a cell with a viral nuclear inclusion |
| Garrote et al., 2015 [3] | 50/M | Visual disturbance, diminished muscular strength in the R arm and vesicular-papular lesions in the L ophthalmic branch region of the V cranial nerve | Chronic lymphocytic leukemia | Fludarabine, cyclophosphamide and rituximab | T2 hyperintensity in bilateral parietal and occipital lobules including internal capsule | Negative PCR for JCV | Infiltration of the brain tissue by foamy macrophages and mature lymphocytes with perivascular clustering |
| Loss of myelin in immunohistochemistry | |||||||
| Reactive astrocytes with polymorphic nuclei and prominent nucleoli |
PCR, polymerase chain reaction; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; F, female; M, male; R, right; L, left; RRMS, relapse and remitting multiple sclerosis; MS, multiple sclerosis; JCV, JCV, John Cunningham virus; EM, electron microscopy; FLAIR, fluid-attenuated inversion recovery; HIS, high signal intensity; AIDS, acquired immune deficiency syndrome; HAART, highly active antiretroviral therapy; NA; not available; NHL, non-Hodgkin lymphoma; GVHD, graft versus host disease; alloHCT, allogeneic hematopoietic cell transplantation; BMT, bone marrow transplantation; HIES, hyper immunoglobulin E (IgE) syndrome; IVIg, intravenous IgG; DLBCL, diffuse large B-cell lymphoma; RCHOP, rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone.
Table 3
| Study | Age (yr)/sex | MRI lesion (T2/FLAIR hyperintensity) | Lesion enhancement | Comorbid diseases and treatment | Treatment for PML | Interval between the initial symptom onset and diagnosis (mo) | Clinical outcome |
|---|---|---|---|---|---|---|---|
| Garrote et al., 2015 [3] | 50/M | Bilateral parietal and occipital lobules, and internal capsule | - | CLL treated with rituximab, fludarabine, cyclophosphamide | Mefloquine and dexamethasone | NA | Marked improvement |
| Shin et al., 2014 [4] | 67/M | R Parietal lobe | - | IgAN on prednisone | Mefloquine | NA | Marked improvement |
| Nishigori et al., 2019 [5] | 68/M | Bilateral MCPs, pons and cerebellum | + | RA treated with prednisolone, and methotrexate for 9 years | Mefloquine and mirtazapine | 6 | Marked improvement |
| Hamaguchi et al., 2020 [6] | 68/M | R MCP and cerebellar hemisphere | + | RA, SLE on prednisolone, tacrolimus | Mefloquine and mirtazapine | 5 | Marked improvement |
| Ishikawa et al., 2018 [7] | 36/M | Bilateral temporoparietal lobe | - | SLE, HLH on prednisolone, cyclosporin A, rituximab, cyclophosphamide, mycophenolate mofetil | Mefloquine and mirtazapine | 3 | Marked improvement |
| Nambirajan et al., 2017 [8] | 44/M | Bilateral parieto-occipital subcortical and deep white matter | - | - | Mefloquine and cotrimoxazole | 1 | Marked improvement |
| Gofton et al., 2011 [9] | 54/F | R cerebellum and brainstem | - | Sarcoidosis on steroid | Mefloquine | 6 | Marked improvement |
| Hervás et al., 2015 [10] | 51/M | Bilateral MCPs and R frontal subcortical white matter | + | RRMS treated with natalizumab | Intravenous methylprednisolone and mefloquine | 1 | Marked improvement |
| Young et al., 2012 [11] | 57/M | R BG, thalamus, R frontal WM | + | HIV on HAART | Mefloquine | 3 | Marked improvement |
| 39/M | R frontoparietal subcortical white matter | - | HIV on HAART | Mefloquine | NA | Marked improvement | |
| Epperla et al., 2014 [12] | 72/M | L frontal lobe | + | CLL s/p splenectomy | Mefloquine and mirtazapine | 1 | Marked improvement |
| Sanjo et al., 2016 [13] | 53/M | L frontal, parietotemporal and R parietal lobes | + | Follicular lymphoma treated with CCRT | Mefloquine, risperidone, and cytarabine | 1.5 | Marked improvement |
| Yoshida et al., 2014 [14] | 40/F | R occipital and L frontal lobes | NA | GVHD treated with calcineurin inhibitor and steroid | Mefloquine and mirtazapine | NA | Marked improvement |
| Yoshida et al., 2015 [15] | 66/M | L frontal lobe | - | Chronic hepatitis C after liver transplantation, GVHD on tacrolimus and rapamycin | Mefloquine | NA | Marked improvement |
| Shirai et al., 2014 [16] | 51/M | L MCP and cerebellar lesion | - | Chronic hepatitis B with hepatocellular carcinoma | Mefloquine and methylprednisolone | 3 | Some improvement |
| 66/F | R frontal lobe | - | SLE, DM, SSc | Mefloquine and mirtazapine | 2 | Some improvement | |
| Hirayama et al., 2011 [17] | 60/M | Bilateral frontoparietal lobe | - | Sarcoidosis | Mefloquine | 4 | Marked improvement |
| McGuire et al., 2011 [18] | 74/F | R frontal lobe | + | Idiopathic isolated CD8+ T-lymphocytopenia | Mefloquine and mirtazapine | NA | Some improvement |
| Ishii et al., 2018 [19] | 37/F | Bilateral cerebral peduncles, internal capsule, corpus callosum, and deep white matter of the L frontal lobe and bilateral periventricular area | - | SLE treated with prednisolone, mycophenolate mofetil | Mefloquine | 5 | Some improvement |
| Ikeda et al., 2017 [20] | 32/F | L frontal lobe | NA | SLE treated with oral prednisolone, tacrolimus and cyclophosphamide pulse | Mefloquine and mirtazapine | 1 | Marked improvement |
| Nakayama et al., 2020 [21] | 73/F | L MCP, cerebellar hemisphere, brainstem | NA | ET treated with ruxolitinib | Mefloquine and mirtazapine | 7 | Some improvement |
| Silverio et al., 2015 [22] | 69/M | L inferior frontal lobe to corona radiata | - | Follicular lymphoma treated by rituximab, Pulmonary sarcoidosis | Mefloquine and mirtazapine | 2 | Some improvement |
| Kurmann et al., 2015 [37] | 56/M | L medial thalamus, hypothalamus, mesencephalon, and tegmentum pontis | - | CVID on IVIg | Mefloquine and mirtazapine | 2 | Marked improvement |
PML, progressive multifocal leukoencephalopathy; MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery; M, male; F, female; CLL, chronic lymphocytic leukemia; NA, not available; R, right; L, left; IgAN, immunoglobulin A nephropathy; MCP, middle cerebellar peduncle; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; HLH, hemophagocytic lymphohistiocytosis; RRMS, relapse and remitting multiple sclerosis; BG, basal ganglia; WM, white matter; HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; s/p, status post operation; CCRT, concurrent chemoradiation therapy; GVHD, graft versus host disease; DM, dermatomyositis; SSc, systemic sclerosis; ET, essential thrombocytopenia; CVID, common variable immune deficiency; IVIg, intravenous immunoglobulin.
References
- TOOLS
-
METRICS

-
- 1 Crossref
- 0
- 5,553 View
- 70 Download
- ORCID iDs
- Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print