Successful treatment with rituximab for central nervous system vasculitis caused by Epstein-Barr virus-associated lymphoproliferative disorder with immunoglobulin M gammopathy
Article information
Abstract
Monoclonal gammopathy of undetermined significance (MGUS) is associated with several autoimmune conditions, including central nervous system (CNS) vasculitis. Epstein-Barr virus (EBV) is a pathogen capable of triggering a systemic immune response and is involved in the occurrence of a wide range of B-cell lymphoproliferative disorders. In systemic autoimmune diseases, EBV infection is suspected to play a central role in pathogenesis. Here, we present a case that was thought to be a systemic autoimmune disease and CNS vasculitis that developed after EBV infection, demonstrating that rituximab is effective for the treatment.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is an asymptomatic premalignant clonal plasma cell or lymphoplasmacytic proliferative disorder [1]. Annually, approximately 1% of MGUS transform to multiple myeloma or other related disorders such as B-cell lymphoma, Waldenstrom’s macroglobulinemia, or immunoglobulin light chain amyloidosis [2]. The exact etiology of MGUS has not been identified; however, the disease may be associated with several autoimmune conditions, including central nervous system (CNS) vasculitis [3]. Epstein-Barr virus (EBV) has been shown to be involved in the occurrence of a wide range of B-cell lymphoproliferative disorders. EBV infection is suspected to play a central role in the pathogenesis and may contribute to the development of autoimmune disease by increasing the proportion of immune cells with autoimmunity [4]. Similarly, autoimmune disease can occur post EBV infection, but reports on the CNS are still not sufficient. Here, we present the case of a 42-year-old woman with CNS vasculitis associated with monoclonal gammopathy of immunoglobulin M (IgM) kappa type after infection with EBV. After steroid treatment, only a partial effect was shown, but rituximab administration caused immediate and complete remission. Rituximab may be considered as a treatment option for patients suspected of having CNS vasculitis following viral infection, particularly EBV infection.
Case Report
A 42-year-old woman was admitted to another hospital due to headache and fever that began 7 days prior to admission. The patient had a 10-year history of episodic paresthesia in her fingers and toes when exposed to cold, which was diagnosed as limited systemic sclerosis 3 years before admission. The patient also had a history of recurrent umbilical hernia and pelvic organ prolapse of unknown etiology. Initial cerebrospinal fluid (CSF) analysis revealed pleocytosis of 459 white blood cells/mm3 (leukocytes 88%; lymphocytes 12%), an elevation protein level of 161 mg/dL, and low glucose (36 mg/dL) with positive EBV DNA polymerase chain reaction (PCR) results. The initial magnetic resonance imaging (MRI) showed multifocal patchy T2 high signal intensity in bilateral cerebral hemispheres with hemorrhagic changes (Figure 1A and B). She was treated with intravenous vancomycin (aim for predose level 15–20 mg/L), ceftriaxone (2 g intravenously [IV] every 12 hours), acyclovir (10 mg/kg), and dexamethasone (10 mg IV every 6 hours). Despite receiving antimicrobial treatment based on the meningoencephalitis diagnosis, the patient’s headache gradually worsened and her visual field began to blur. She was referred to our hospital for further evaluation after 7 days of antimicrobial treatment.
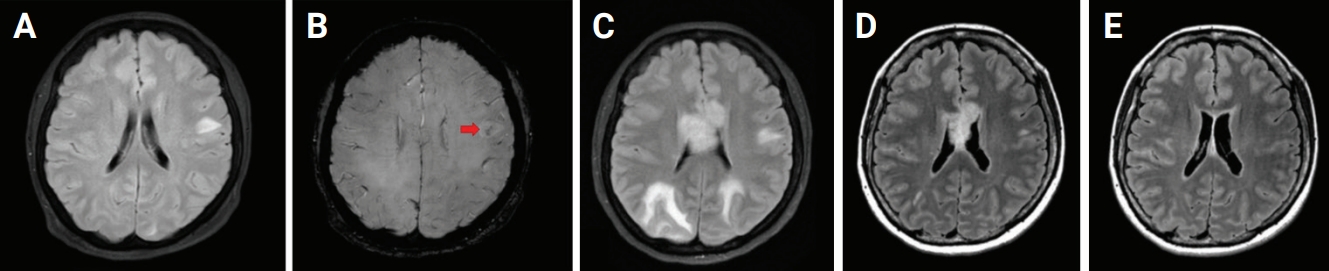
Serial brain magnetic resonance images of the patient
(A) A 4.5-cm patchy T2 high signal intensity (SI) in the left hemisphere. (B) A 4.5-cm size lesion with inner hemorrhagic change transformation on susceptibility-weighted imaging in the same location (red arrow). (C) Interval increased in the extent of multifocal patchy T2 high SI along the bilateral cerebral hemispheres and corpus callosum. (D) Decrease in multifocal patchy T2 high SI along the bilateral cerebral hemispheres and corpus callosum after the 4th cycle of rituximab. (E) Further decrease in multifocal patchy T2 high SI along the bilateral cerebral hemispheres and corpus callosum after the 11th cycle of rituximab.
Upon physical examination, neither the liver nor spleen were palpable in the abdomen, and there was no edema of the lower extremities. Neurological examination revealed a drowsy mentality, general weakness (MRC [Medical Research Council] grade III), and decreased visual acuity to differentiate only hand motion. Vital signs at initial evaluation were: blood pressure, 119/87 mmHg; heart rate, 64 beats/min; respiratory rate, 18 breaths/min; and temperature, 37.3℃. In the blood test, the hemoglobin sedimentation rate was 56 mm/hr, the C-reactive protein was 3.09 mg/dL, serum cryoglobulin was positive, and mild hyponatremia (133 mEq/L) was observed. Except for the above, no abnormal findings such as anemia were observed in the blood tests. In the complete blood count, leukocytes were 2,660/μL, hemoglobin was 8.6 g/dL, platelets were 213,000/μL, mean cell volume was 80.3 fL, and mean corpuscular hemoglobin was 23.6 pg. Biochemical tests found that the total protein was 10.2 g/dL, albumin was 3.26 g/dL, aspartate aminotransferase was 24 IU/L, alanine aminotransferase was 19 IU/L, alkaline phosphatase was 52IU/L, lactic dehydrogenase was 186 IU/L, total bilirubin was 0.6 mg/dL, blood urea nitrogen was 12 mg/dL, and creatinine was 0.89 mg/dL. Serum electrolytes were Na 133 mEq/L, K was 4.4 mEq/L, Cl was 112 mEq/L, total calcium was 8.4 mEq/L, ionized calcium was 4.63 mEq/L, uric acid was 4.3 mg/dL, serum cholesterol was 94 mg/dL, and serum triglyceride was 24 mg/dL; proteinuria was not observed as a result of urinalysis.
CSF analysis was conducted in our hospital and showed 32 white blood cells/mm3 (leukocytes, 12 cells/µL; lymphocytes, 12 cells/µL; and others, 8 cells/µL), 40 red blood cells/mm3, 138 mg/dL of protein, and 33 mg/dL of glucose (serum glucose, 99 mg/dL). The opening pressure was normal at 17 cm H2O.
No specific antibodies against neuronal cell-surface or synaptic proteins were found in the serum or CSF. In addition, viral PCR in the CSF and serum were all negative, including EBV. Serum protein electrophoresis was normal (total protein, 7.0 g/dL; M-spike, 0%), but immune-typing results showed a dimmed monoclonal band against the anti-IgM and anti-kappa antiserum suggesting monoclonal gammopathy of the IgM kappa type. There was no evidence of clonal plasma cell infiltration in bone marrow biopsies. MRI findings showed aggravated multifocal patchy T2 high signal intensity in bilateral cerebral hemispheres compared with the previous study (Figure 1C). The positron emission tomography scan revealed relatively mild hypometabolic lesions in the left high parietal and right occipital cortices, suggesting CNS inflammation (Figure 2). Conventional angiography showed stenoses of multiple medium-sized brain arteries in the form of beads on string, a typical finding of CNS vasculitis (Figure 3). The patient received a high-dose corticosteroid based on the clinical diagnosis of CNS vasculitis. After steroid administration, visual acuity and motor power recovered quickly, but the patient continued to complain of gait imbalance. Intravenous rituximab (375 mg/m2) was administered at regular intervals (the first four sessions were administered weekly and then monthly thereafter). By the end of the fourth cycle, the patient’s clinical symptoms had recovered completely and image findings were improved (Figure 1D). The CSF profile was also improved with 3 white blood cells/mm3, as well as a normal range of protein (40 mg/dL) and 62 mg/dL of glucose (serum glucose, 127 mg/dL). Rituximab was maintained on a monthly schedule, and the patient remained symptom-free for over a year. Furthermore, the T2 high signal seen on MRI almost disappeared after the 11th cycle of rituximab (Figure 1E).

F-18 fluorodeoxyglucose positron emission tomography of the brain
Relatively mild hypometabolic lesions in left high parietal and right occipital cortices (round dotted line).
Discussion
We present a case of CNS vasculitis that is presumed to have developed after EBV infection. At first, brain involvement of systemic sclerosis was considered due to past medical history. However, the characteristic brain involvement of systemic sclerosis, such as small vessel calcification and intracerebral calcification [5], was not observed. Cryoglobulin-related vasculitis was also considered but was excluded after observing that angiography showed the vasculitis invading medium-sized blood vessels and not small blood vessels and because it responded well to steroids [6].
Although the precise etiology of CNS vasculitis is unknown, infectious agents have been proposed as triggers [7]. EBV is a pathogen capable of triggering a systemic immune response, including in the CNS [8]. EBV is a human herpes virus, and 90%–95% of the adult human population carries EBV as a chronic latent infection [4]. Most EBV infections are asymptomatic, but EBV sometimes causes a systemic infection or reactivation that may directly involve the CNS [9]. The nervous system is clinically involved in EBV infection in 0.5%–7.5% of individuals, and the most common CNS complications of EBV infection include encephalitis, cerebellitis, meningitis, cranial nerve palsies, and myelitis [7]. However, there are few reports of CNS vasculitis associated with EBV infection. Unfortunately, treatment has not yet been established.
In our case, CSF pleocytosis persisted even after antimicrobial treatment, and considering the negative conversion of EBV DNA, the most likely explanation is that CNS vasculitis occurred after EBV infection. Kim et al. [10] recently reported a patient who was presumed to have developed acute disseminated encephalomyelitis after EBV infection and was treated with rituximab after failing steroid and IV immunoglobulin therapy. As in our case, in the above study, EBV DNA was converted to negative, and the MRI image improved after treatment with rituximab. The pathological development of EBV-related neurological diseases can be immune-mediated, infected, or both [11]. EBV infection may directly or indirectly contribute to the development of the pathogenesis of EBV-associated vasculitis by an immune-mediated reaction. EBV primarily targets B cells via interaction of the viral envelope glycoprotein, and EBV activates primary human B lymphocytes [12]. The preference of EBV for B cells may explain why the anti-CD20 monoclonal antibody rituximab, which targets B cells, has resulted in significant clinical improvements.
Several studies demonstrated a higher incidence of EBV positivity in lymphoproliferative disorder patients than in the general population [13]. A small percentage of these carriers, particularly those with immunodeficiency, develop EBV-positive lymphoproliferative disorders, even though some disorders also develop in the general population [13]. In adults, EBV-positive lymphoproliferative disorders may be caused by dysregulation of the immune response to EBV infection, reduced immunity with aging, and iatrogenic immune suppression [14]. We believe that several autoimmune responses followed sequentially due to changes in immune responses after EBV infection.
Monoclonal gammopathy, whether malignant or of undetermined significance (MGUS), results from clonal proliferation of differentiated plasma cells producing homogeneous whole immunoglobulin or light chain [15]. Reactivation of EBV has been implicated in the pathogenesis of monoclonal gammopathy [16]. Excessive production of abnormal clonal gamma globulins, or paraproteins, causes changes in the circulation by increasing the viscosity [17]. Several studies have been conducted to determine the predictive value of the M protein as a marker of lymphoid proliferations disease [18], but further studies are still needed.
The patient presented here also had a history of pelvic organ prolapse and umbilical hernia, suggesting that she may have had a connective tissue disorder. Systemic autoimmune diseases (SADs) are a group of connective tissue diseases with diverse, yet overlapping, symptoms and autoantibody development [4]. Because of the relationship between SADs and MGUS, we tested whether the gene was involved or not, but there were no special abnormalities, and no one in the family showed similar symptoms.
In conclusion, we report a lymphoproliferative disorder that appeared after EBV infection in the form of systemic sclerosis and CNS vasculitis. Steroids can be used as first-line treatment, but if the response to steroids is limited, rituximab can be considered as another treatment option for the patient.
Notes
Conflicts of Interest
Kon Chu has been on the editorial board of encephalitis since October 2020. He was not involved in the review process of this case report. No other potential conflicts of interest relevant to this article are reported.
Author Contributions
Conceptualization: Chu K; Visualization: Lee J; Supervision: Chu K, Lee HS; Writing-original draft and editing: Lee J; Writing-review and editing: all authors.

