Posttransplant lymphoproliferative disorder in a kidney transplant recipient: a case of Epstein-Barr virus-positive primary central nervous system lymphoma
Article information
Abstract
Posttransplant lymphoproliferative disorders (PTLDs) are potentially life-threatening complications of chronic immunosuppression in patients who receive solid organ transplants or allogeneic hematopoietic cell transplantation. Most PTLD cases are associated with Epstein-Barr virus (EBV) serology, and their incidence is typically higher in the first year of transplantation. Isolated EBV-positive diffuse large-cell B-cell lymphoma in the renal transplant setting has rarely been reported. Isolated EBV-positive primary central nervous system lymphoma (PCNSL) is rare, even in renal transplant patients with chronic immunosuppression. We report a case of frontal lobe EBV-positive PCNSL in a renal transplant patient who presented with left-sided weakness and was later treated with a consolidated chemotherapeutic regimen without concurrent radiotherapy.
Introduction
Posttransplant lymphoproliferative disorders (PTLDs) involve B-lymphocytes that are elevated in the environment of chronic immunosuppression in cases of solid tumor or allogeneic hematopoietic cell transplant (HCT) [1]. In most cases of PTLD, Epstein-Barr virus (EBV)-driven B-cell proliferation occurs due to reduced activity of T cells. Immunocompetent individuals can cause a cytotoxic T cell response that eliminates EBV-infected B cells. Still, a small number of infected B cells downregulate viral antigen, which helps them to evade immune surveillance. These can persist as a latent infection; in immunosuppression, they can also cause reactivation, which results in PTLD [2]. PTLD is related to the degree and type of immunosuppression and the donor or recipient EBV serology at the time of transplantation [3]. The incidence of EBV positivity in renal transplant patients is up to 3%, with a greater incidence in the first year after transplantation [1,4]. Isolated cases of PTLD-related, EBV-positive primary central nervous system lymphoma (PCNSL) are rare, and the presentation can vary depending on the lymphoma location. We report a rare case of isolated EBV-positive PCNSL that came to our attention due to insidious left-sided weakness.
Case Report
A 62-year-old female presented to our tertiary care facility with complaints of progressive generalized weakness and recent falls for the past 1 week. The patient had a previous medical history of cadaveric renal transplantation 16 years ago and was currently on immunosuppressive therapy (tacrolimus 6 mg, mycophenolate 360 mg, and prednisone 360 mg; once daily, respectively); she also had a history of hypertension and subtotal parathyroidectomy. On presentation, her vitals were stable. The physical examination was significant for generalized moderate gross motor weakness without sensory deficits and isolated left upper extremity weakness. The ophthalmological exam was unremarkable. The patient also reported paravertebral lower back pain and neck pain. Although the patient reported that the weakness was not new, a brain computed tomographic scan was carried out due to suspicion of stroke, which was a concern for an enlarging parenchymal mass within the left frontal lobe. Brain magnetic resonance imaging (MRI) with contrast was performed to characterize the lesion further and revealed a peripherally enhancing, 2.8-cm, left frontal lobe lesion with surrounding edema and mass effect (Figure 1). An MRI of the spine reported multilevel cervical spondylosis and myelomalacia at C5-C6, which explained the patient’s isolated left-sided gross motor power changes. In the past 6 months, the patient reported an approximately 27 kg unintentional weight loss without night sweats or fevers. She did not report any personal history of personality changes, but the family reported slowness of thought and “increased quietness” during the last two months. Infectious disease and neurosurgery departments were consulted. Multiple infectious etiologies were investigated, and the serum was positive for toxoplasma immunoglobulin G and John Cunningham (JC) virus DNA. Infectious disease and neurosurgery recommended an excisional biopsy; based on their input, toxoplasmosis was unlikely to be the cause of presenting symptoms, and the lesion was not classical for the JC virus. The patient underwent excisional biopsy via craniotomy, and the pathology was consistent with PCNSL: EBV-positive diffuse large B-cell lymphoma (DLBCL), non-germinal center (GC) type (Figure 2).

Magnetic resonance imaging
(A) T1-sequence showing a 27.7 × 22.5 mm peripheral enhancing lesion (arrow) in the frontal cortex. (B) Fluid-attenuated inversion recovery showing a peripherally enhancing lesion with surrounding edema. (C) Diffusion-weighted image.

Histopathological images of brain biopsy
Neoplastic cells were centered around blood vessels (arrows) with necrotic cells (pink areas) surrounding aggregates of viable cells. Cytologically, the neoplastic cells had moderate-sized nuclei and scant but discernible cytoplasm. H&E staining; A, ×50 and B, ×100.
Fluorescence in situ hybridization analysis was negative for MYC amplification or rearrangement and BCL6 and BCL2 rearrangement. A t(8;14) translocation was not detected (Table 1). The analysis identified no evidence of a “double” or “triple” hit lymphoma. Likewise, there was no evidence of a Burkitt lymphoma-related translocation. Findings confirmed the immunohistochemical impression of an activated B-cell–like DLBCL (Figure 3).
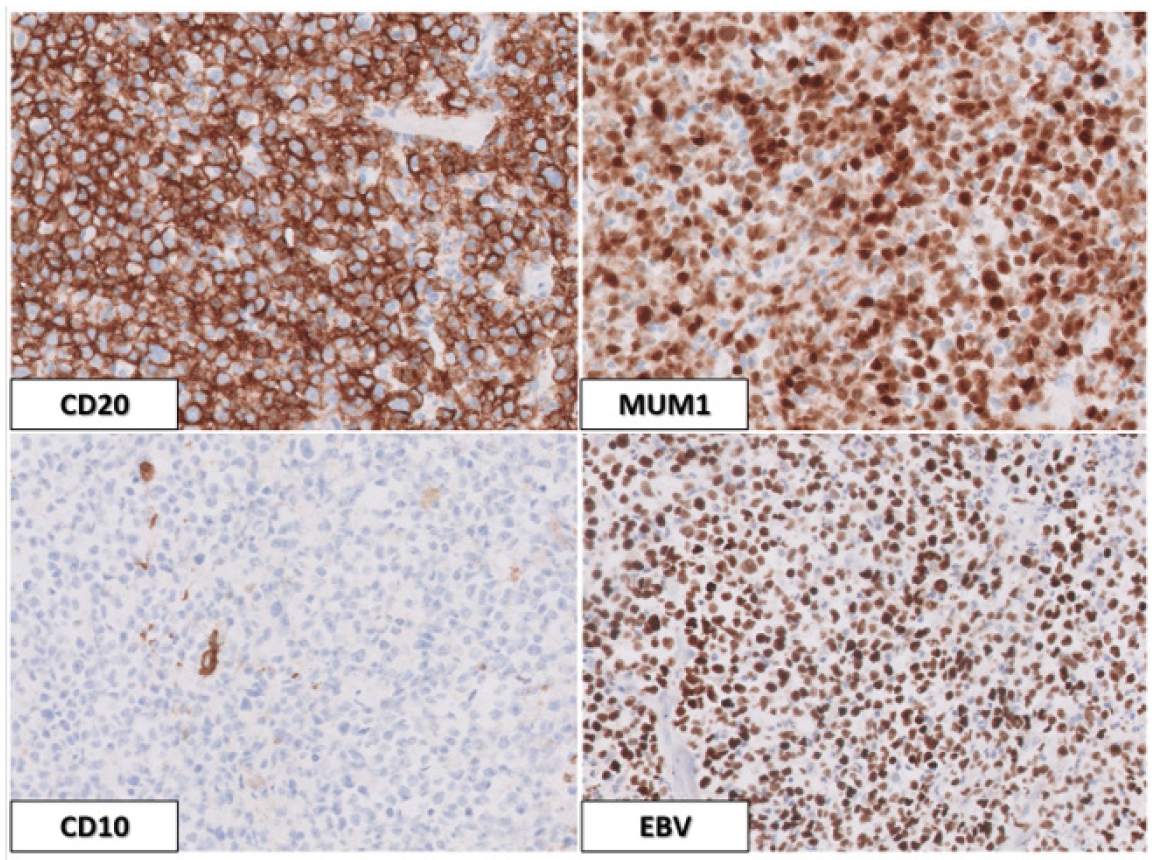
Immunohistochemical evaluation of neoplastic lesion
(A–C) An immunohistochemical evaluation revealed that neoplastic cells showed strong membranous expression of CD20 and nuclear expression of MUM1 but lacked expression of CD10. Most neoplastic cells also showed expression of BCL-2 and PAX-5, while over 30% of cells showed BCL-6 expression (not shown). The Ki-67/MIB-1 proliferation index was very high at 30% to 50% (not shown). Admixed T cells showed expression of CD3 and CD5, while neoplastic cells lacked expression of these antigens (not shown). (D) By in situ hybridization, Epstein-Barr virus (EBV) expression was noted in most neoplastic cells. The combined findings were consistent with a designation as a diffuse large B-cell lymphoma of the non-germinal cell type that was EBV-positive. A–D, ×50.
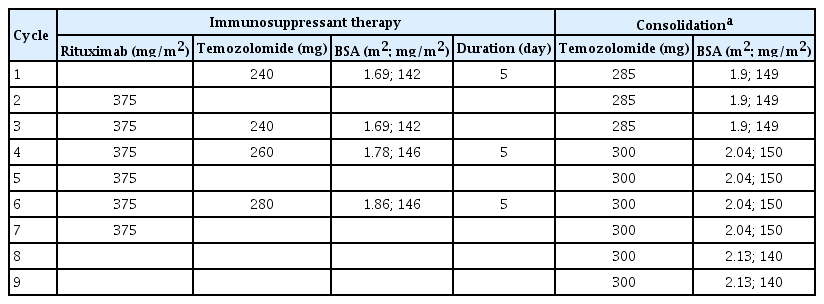
Lumbar puncture was unremarkable for flow cytometry and cytopathology. Oncology suggested five cycles of chemotherapy with rituximab, methotrexate, procarbazine, and vincristine, and the patient was discharged to outpatient follow-up within 1 month. High-dose methotrexate (HD-MTX)-based treatment was not administered due to severe renal dysfunction (24-hour creatinine clearance of 30 mL/min). The patient is currently on rituximab and temozolomide. The doses of chemotherapeutic drugs that were administered during each cycle are shown in Table 2. During subsequent follow-up visits, the patient’s constitutional weakness and bradyphrenia improved, and she underwent consolidation chemotherapy (Table 2).
Discussion
PTLD is a plasmocytic proliferation resulting from immunosuppression in the setting of solid organ transplantation. In most cases, PTLD is an EBV-positive B-cell proliferation due to reduced T cell immune surveillance from immunosuppression [2]. Acute EBV infection leads to polyclonal expansion of B cells that harbor the EBV. A small subpopulation of infected B cells can escape immune surveillance by downregulating viral antigen expression [1-4]. During chronic immunosuppression, these infected B cells give rise to lymphoproliferative disorders, such as PTLD. Primary central nervous system (CNS) PTLD is uncommon, and the present study reports a case of frontal lobe EBV-positive PCNSL in a renal transplant patient who presented with left-sided weakness and was later treated with a consolidated chemotherapeutic regimen with significant improvement [2-4]. PCNSL is a type of non-Hodgkin’s lymphoma that accounts for 0.3% to 1.5% of all intracranial neoplasms and 0.7% to 2% of lymphomas and is associated with underlying immunosuppression [5]. PCNSL can occur in the brain, eyes, or spine, and there is little evidence of systemic spread [6]. Patients usually present with neurological signs within weeks, including focal neurologic deficits, mental status changes, and behavioral alterations related to increased intracranial pressure, including papilledema, headache, and seizures [7]. The initial stage of clinical presentation varies depending on the area involved. Many of the patient’s presenting symptoms were behavioral and neurocognitive changes, which are nonspecific and can delay the diagnosis. Compared to other space-occupying lesions, patients can present with raised intracranial pressure and should be evaluated over time. Patients might present with ocular symptoms, including decreased visual acuity, blurred vision, or floaters. The clinical presentation in a patient with intramedullary spinal involvement depends on the area of the spine occupied by the tumor; however, the thoracic spine is typically involved. Leptomeningeal involvement can present with cerebrospinal fluid dissemination and parenchymal lesions [7].
In contrast to other primary or metastatic brain tumors, PCSNL usually occupies subcortical and deep structures and spares the cerebral cortex. Thus, seizures are less likely to occur in these patients [8]. Patients typically do not present with constitutional B symptoms, such as night sweats, fever, and weight loss, which are typical in non-Hodgkin’s lymphoma [9]. This pattern was demonstrated by our case where the patient did not report any B symptoms.
Diagnosis of PCNSL requires a high degree of suspicion upon presentation. MRI with contrast can show a ring-enhancing lesion, and brain biopsy with histopathologic evaluation can confirm the pathology. Characteristic imaging findings typically reveal a uniform contrast-enhancing mass with vasogenic edema, which produces a mass effect in the lateral ventricles. After confirmation of the diagnosis, a thorough systemic evaluation should involve the eyes, spine, and systemic organs. This can help determine the therapy of choice and reveal the extent of the disease [10]. Patients with PTLD involving the CNS should be considered for treatment with the protocols used for PCNSL [11]. First-line treatment of PCNSL consists of chemotherapy followed by consolidation via whole-body radiation therapy [7]. A complete radiological response is recommended before starting consolidation therapy for PTLD-related PCNSL. Whole-brain radiation therapy (WBRT), high-dose chemotherapy with autologous HCT, or nonmyeloablative chemotherapy is considered for consolidation based on patient profile and toxicities of certain regimes [12]. Despite the initial high response rates of PCNSL to steroids, the radiographic and clinical improvement is only transient and tends to recur. Therefore, these modalities are used for supportive therapy only [13]. Steroid use is discouraged before biopsy as it can cause false remission and interfere with the tumor’s histopathology because of its lymphocytotoxicity. Another risk is opportunistic infections while a patient is undergoing chemotherapy and is on high-dose steroids. Therefore, prophylactic antibiotics should be considered.
The treatment of PTLD-related EBV-positive PCNSL presents a significant problem. The conventional treatments that can work for PCNSL might not be as effective in the EBV-positive variant, as demonstrated by the retrospective study results of Park et al. [14], which showed an inadequate response and a worse survival rate in EBV-positive lymphomas compared to EBV-variants. Whole-body radiation therapy has been used for years as a first-line treatment for EBV-positive PCNSL. While the initial response appears promising, it is associated with significant neurotoxicities, like cognitive impairment, deafness, behavioral disturbances, incontinence, and weakness [15]. Raez et al. [16] reported a positive response in four immunosuppressed patients with AIDS-related EBV-positive lymphoma who were treated with azathioprine and zidovudine ganciclovir interleukin-2.
The positive response to antiretrovirals in these patients is due to viral kinases, which successfully phosphorylated the drugs and led to an efficacious response. Roychowdhury et al. [17] hypothesized that induced expression of EBV thymidine kinase and other lytic gene products would support the phosphorylation of azathioprine and ganciclovir, thereby enhancing the cytotoxic activity against EBV-positive PCNSL in vivo. Further studies and clinical trials are warranted to study these inductions in successful treatment of these patients.
Comorbidities can be a hurdle in forming treatment plans due to the often extensive side effect profiles of chemotherapeutic agents. HD-MTX was initially selected for our patient but was not administered due to her existing severe renal dysfunction (24-hour creatinine clearance of 30 mL/min). She was then started on rituximab and temozolomide. An antiviral regimen can also be used, as demonstrated by Hansen and Nielsen [18] with their initial use of ganciclovir followed by valganciclovir. In most cases, an immunotherapy dose reduction, steroids with chemotherapy, and radiotherapy were used; the chemotherapy choice depended on clinician and side effect profiles [18-24]. Conservative therapy here is defined by an immunotherapy dose reduction or forgoing chemotherapy or radiotherapy. This approach was employed by Valavoor et al. [19] and Mazanowska et al. [23] due to patient comorbidities and intolerability.
Posttransplant EBV-positive DLBCL in CNS is a rare and aggressive disease with a poor prognosis, especially in the elderly. Based on the classification proposed by Hans et al. [25], B-cell lymphoma could be GC B-cell lymphoma (CD10 positive) or non-GC–activated B-cell–like DLBCL (CD10 negative) (Figure 4).
We discussed a case where a frontal lobe PCNSL was coincidentally found on imaging, and the retrospective history revealed symptoms that otherwise would have evaded early diagnosis for many months. A particular focus should be placed on neuropsychiatric symptoms when obtaining a history, and a concurrent family history should be performed. Depression, apathy, confusion, memory impairment, or slowness of thought can present as symptoms of PTLD-related PCNSL and should raise a red flag in patients with clinical suspicion. Diagnosis should always be confirmed by biopsy, and treatment mostly consists of chemotherapy followed by WBRT and immunotherapeutic reduction. However, cases can vary. While antiviral prophylaxis can decrease the incidence of PTLD-related PCNSL, antiviral therapy has yet to establish convincing efficacy for treating PCNSL.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Ali H; Project administration: Ali H; Investigation, Resources: Bin Waqar SH, Majeed M, Sehar A, Mumtaz A; Supervision: Ali H; Writing–original draft: Majeed M, Bin Waqar SH, Sehar A, Mumtaz A; Writing–review and editing: Ali H
Acknowledgements
We thank the patient for providing authorization for medical record use.