A case of hyperacute postvaccinal encephalopathy after BNT162b2 nCoV-19 vaccine
Article information
Abstract
The global severe acute respiratory syndrome coronavirus 2 pandemic contributed to the development of a large variety of vaccines, of which postvaccinal hyperacute encephalopathy is a very rare complication. Despite its rarity, if diagnosed properly, appropriate treatment can be rapidly applied. A healthy 53-year-old woman was admitted for a seizure on the day she received the second dose of the BNT 162b2 nCoV-19 vaccine. She subsequently developed irritability, which gradually worsened over several days. Cerebrospinal fluid analysis revealed mild pleocytosis and normal protein levels. Brain magnetic resonance imaging (MRI) revealed diffuse sulcal hyperintensity on the entire brain surface on fluid-attenuated inversion recovery images with meningeal enhancement. The patient was diagnosed with hyperacute postvaccinal encephalopathy and received immunosuppressive therapy with corticosteroids and therapeutic plasmapheresis. Fortunately, the patient responded to therapy, achieving almost complete recovery from the neurological symptoms, with only mild memory impairment remaining after 3 weeks. Based on the clinical presentation, electroencephalogram findings, and MRI, our patient developed hyperacute encephalopathy within 24 hours of vaccine administration, which we surmised from the temporal course of symptoms and brain imaging findings. Further studies are required to elucidate the pathogenesis of coronavirus disease 2019 vaccination-related encephalopathy.
Introduction
The global severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic contributed to more than 525,983,796 confirmed infections and 11,312,690 deaths worldwide according to the World Health Organization. The severity of the pandemic led to unexpected development of a variety of vaccines. Due to excellent safety and efficacy data from clinical trials, the BNT162b2 nCoV-19 vaccine (Pfizer-BioNTech) was approved. A growing number of recent reports of autoimmune-induced thrombocytopenia and thrombosis in addition to local and systemic side effects of the BNT 162b2 nCoV-19 vaccine have raised concerns.
Herein, we present a case of hyperacute reversible encephalopathy related to prior BNT162b2 nCoV-19 vaccine. Such a case has not been previously described. The timeline of its clinical course, diagnostic investigations, and therapies are shown in Figure 1.
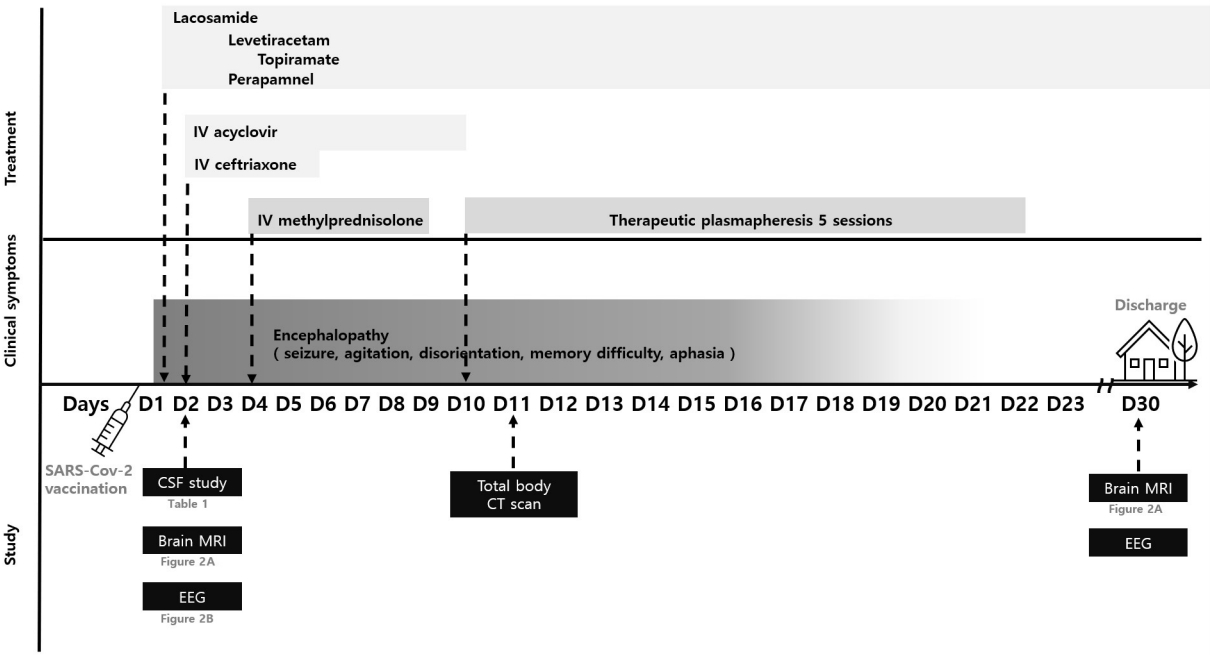
Time trends in clinical events and diagnostic investigations
IV, intravenous; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CSF, cerebrospinal fluid; EEG, electroencephalogram; MRI, magnetic resonance imaging; CT, computed tomography.
This study was approved from the Institutional Review Board of the Dogguk University Ilsan Hospital (No. DUIH 2023-07-029). Written informed consent was obtained for publication of this report and accompanying images.
Case Report
A healthy 53-year-old woman was admitted for a seizure occurring on the day she received the second dose of the BNT 162b2 nCoV-19 vaccine. The patient had received the first dose of the BNT 162b2 nCoV-19 vaccine without noticeable side effects 12 weeks previously and had no history of neuropsychiatric disease. Neurological examination revealed disorientation, slurred speech, and memory loss in the absence of meningeal irritation and other neurological signs. The patient developed irritability, which gradually worsened over several days. Laboratory results, including blood count, erythrocyte sedimentation rate, C-reactive protein, and kidney and liver function, were normal. SARS-CoV2 polymerase chain reaction (PCR) tests using nasopharyngeal swabs were negative. Infectious serum screening was negative for herpes and human immunodeficiency virus. Anti-myelin oligodendrocyte glycoprotein antibodies, anti-nuclear, anti-extractable nuclear antigens, anti-neutrophil cytoplasmic, and anti-cardiolipin antibodies were all negative. Cerebrospinal fluid (CSF) analysis (Table 1) revealed mild leukocytosis (eight leukocytes, 16% neutrophils) and normal protein levels. CSF cytology revealed no evidence of tumor cells, and no infectious agents (including mycobacteria) were detected in the culture or by PCR testing for herpes viruses, enterovirus, or meningoencephalitis panel for bacteria and fungi. CSF immunoelectrophoresis revealed normal results, with PCR in the CSF and serum samples. All tested tumor markers were negative, as were the results for blood and CSF antineuronal antibodies including GAD65, NMDA, GABA-B receptor, IgLON5, AMPA-R subtype 2, DPPX, LGI1, CASPR2, glycine receptor, mGluR5, mGluR1, amphiphysin, CV2/CRMP5, Ma2/Ta, Hu, Ri, Yo, Zic4, recoverin, Sox1, and titin. Total body computed tomography revealed no obvious abnormalities. However, brain magnetic resonance imaging (MRI) revealed diffuse cisternal and convex hyperintensity on the fluid-attenuated inversion recovery (FLAIR) images of the entire brain surface, with diffuse meningeal enhancement on contrast-enhanced FLAIR images (Figure 2A). Continuous electroencephalogram (EEG) recordings (Figure 2B) showed ictal activity of theta rhythmic build up arising from the right temporal area.

MRI and EEG findings in a case with hyperacute post-vaccinal encephalopathy
(A) Contrast-enhanced fluid-attenuated inversion recovery (FLAIR) images at admission and 1-month follow-up. Representative axial contrast-enhanced FLAIR images on admission. Diffuse sulcal hyperintensity is shown on FLAIR imaging at the cerebral convexity with diffuse meningeal enhancement on contrast-enhanced FLAIR images. There was no significant imaging evidence of acute disseminated encephalomyelitis. Contrast-enhanced FLAIR images at 1-month follow-up showed remarkable interval improvement of prior diffuse meningeal enhancement. Edematous hyperintensity lesions (white arrows) in both the amygdala and hippocampi were newly developed. (B) Representative electroencephalographic recordings using double banana Montage (sensitivity 10 μV/mm, filter 1 to 70 Hz; the interval between two vertical lines: 1 second). EEG reveals ictal activity theta rhythmic build up arising from the right temporal area (black arrow).
MRI, magnetic resonance imaging; EEG, electroencephalogram.
Initially, the patient was treated with 550 mg of intravenous acyclovir, three times daily, for 7 days and 2 g of ceftriaxone, twice daily, for 3 days until other viral antibody titers were negative. Concomitant antiepileptic drug therapy was initiated. However, the patient’s disorientation and aggressiveness worsened.
Under a presumptive diagnosis of autoimmune encephalitis, we administered a daily dose of 1 g methylprednisolone over a period of 5 days. Unfortunately, the patient remained consistently disoriented and only partially complied with simple tasks without an adequate response. Five sessions of therapeutic plasmapheresis were performed every other day, leading to recovery of consciousness. The patient exhibited improvement in neuropsychiatric symptoms the day after the fourth plasmapheresis. At discharge, EEG revealed relatively frequent spikes in the left frontal lobe and persistent mild cognitive decline and memory difficulties.
One month after discharge, the patient experienced only mild memory impairment, which subsequently improved enough for her to resume daily activities without recurrent seizures. On the follow-up MRI, edematous T2 hyperintensities had newly developed in both the amygdala and hippocampus without abnormal enhancements. Previous abnormal meningeal signal intensity and leptomeningeal enhancement were remarkably improved (Figure 2A).
Discussion
Few cases of hyperacute encephalopathy from vaccination are reported among USA’s Vaccine Adverse Event Reporting system. In our case, the patient began to develop neurological symptoms including seizure, forgetfulness, and mood disturbance within 24 hours after receiving the second coronavirus disease 2019 (COVID-19) vaccine dose, in the absence of other documented etiologies. Clinical presentation, EEG findings, and MRI revealed the development of hyperacute encephalopathy within 24 hours of vaccine administration. Initially, antibiotics and antivirals were immediately administered with the possibility of infectious meningoencephalitis. However, despite receiving ceftriaxone (to treat bacterial meningitis) and intravenous acyclovir (to treat herpes encephalitis), her condition deteriorated further. Fortunately, the patient responded to immunosuppressive therapy with corticosteroids and therapeutic plasmapheresis and exhibited a benign disease course with almost complete recovery from neurological symptoms, and only mild memory impairment remaining. Follow-up MRI suggested a monophasic disease course, exhibiting improved meningeal enhancement. New findings of edema and hyperintensity in both the amygdala and hippocampus suggest the possibility of some form of limbic encephalitis. Considering the patient’s clinical improvement, the emergence of newly observed lesions on imaging studies during hospitalization was postulated. Although these lesions display typical imaging findings of autoimmune limbic encephalitis [1], this case cannot be classified as such due to the rapid onset of symptoms within 24 hours, an insufficient amount of time for autoantibody production. The patient’s dramatic response to methylprednisolone and plasmapheresis may indicate an immune-mediated mechanism underlying her condition. In prior cases, therapeutic plasmapheresis has been used to eliminate cytokines and inflammatory factors in cytokine storms with proven effectiveness [2].
The results of CSF analysis, neuroimaging, and clinical responses to immunosuppressants were consistent with immune-related pathogenesis involving the brain, rather than a direct infectious process targeting the brain. Unfortunately, we did not measure the patient’s cytokine levels. Based on clinical manifestations and the short temporal association with vaccination, a cytokine-mediated inflammatory process has been proposed as a key pathophysiological mechanism for COVID-19–related encephalopathy, also termed cytokine storm-associated encephalopathy [3]. Although the etiology of hyperacute encephalopathy is not fully understood, it could be attributed to different potential mechanisms of vaccine-induced autoimmune disease. It has been hypothesized to be related to factors such as a cytokine storm or other immune-mediated mechanisms triggered by the vaccination [3]. It is plausible to speculate that a cytokine storm may potentially arise as a result of an exaggerated innate immune response triggered by various factors. These factors could include the presence of SARS-CoV-2 coding messenger RNA or DNA, viral vectors, or adjuvants used in vaccines [4,5]. Additionally, in individuals with a documented predisposition to autoimmunity and prior exposure to SARS-CoV-2, it is conceivable that an uncontrolled immune response may be fostered [4,5].
Our patient did not present with any neurological symptoms after the first dose of the BNT162b2 nCoV-19 vaccine. Interestingly, prior studies have indicated that subjects receiving the second dose of BNT162b2 exhibit significantly more severe reactions than after the first dose of the vaccine [6]. The magnitude of the immune response is typically increased by multiple administrations of vaccine because of immunological memory [7]. Several weeks after the first immunization (prime), memory B cells undergo the germinal center reaction over several months [8]. Therefore, it has been suggested that an interval of at least 2 to 3 months between the prime and boost is necessary to obtain optimal responses [8]. With such an immune mechanism, the cytokine storm could represent an excessive innate immune response at the second vaccination in our patient, as the patient had been exposed to the BNT162b2 nCoV-19 vaccine 12 weeks prior. Clinicians may need to be more careful about the occurrence of encephalopathy caused by such hyperimmune reactions due to the nature of the nCoV-19 vaccination, which requires several booster shots, especially when administered in a short period of time.
As nCoV-19 vaccinations have become more common, reports of related side effects are expected to increase. Although rare, fatal neurological side effects require prompt and appropriate treatment.
In the present patient, we based our theory regarding the underlying mechanisms mainly on the temporal course of symptoms and brain imaging findings. Further studies are required to elucidate the pathogenesis of COVID-19 vaccination-related encephalopathy.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Yoon SJ, Kim KK, Kim HR; Supervision: Kim KK, Kim HR; Writing–original draft: Yoon SJ; Writing–review & editing: Yoon SJ, Kim HR, Lee EJ
Acknowledgements
The authors would like to thank Mr. Jae Woo Chung at the Department of Laboratory Medicine, Dongguk University Ilsan Hospital for his kind and excellent performance on therapeutic plasmapheresis.

