Chronic cryptococcal meningitis with a cryptococcoma presenting as normal pressure hydrocephalus: a case report
Article information
Abstract
Chronic meningitis may present with clinical features related to hydrocephalus. We report a 76-year-old female who presented to an outpatient clinic with cognitive decline and gait disturbance with recurrent falls. The initial diagnosis of normal pressure hydrocephalus (NPH) was based on the clinical symptoms and magnetic resonance imaging (MRI) of the brain, which showed ventriculomegaly without an obstructive lesion. During follow-up, however, there was remarkable cognitive decline, and she was unable to walk without assistance. Lumbar puncture and brain MRI showed respective lymphocyte-dominant pleocytosis that was positive for cryptococcal antigen and a new encapsulated abscess-like lesion in a left caudate head. Treatment for cryptococcal meningitis was initiated, and the patient was cured after a long treatment with an antifungal agent. As chronic meningitis could be misdiagnosed as NPH, differential diagnoses of etiologies that can cause hydrocephalus should be addressed.
Introduction
Normal pressure hydrocephalus (NPH) arising in adulthood is characterized by a typical combination of clinical and radiological findings. The classic clinical features of NPH include progressive gait disturbances, urinary incontinence, and cognitive impairment [1].
The typical radiological finding of NPH is ventricular dilatation, which is a common sign of other diseases associated with an increase in intracranial pressure (ICP), such as mass-like lesions, secondary atrophy due to autoimmune diseases or chemotherapy, and chronic inflammation [2]. In the elderly, clinical features of NPH such as cognitive decline are non-specific and can be found in other conditions such as dementia [1]. Thus, the differential diagnoses of NPH features should be excluded when evaluating patients with suspected NPH.
Chronic meningitis may present with clinical features related to hydrocephalus. Chronic meningitis is clinically differentiated from acute and subacute meningitis based on underlying cause; endemic fungi and noninfectious etiologies are more common in chronic meningitis [3]. As patients may have trouble clarifying the onset of vague symptoms that occurred weeks before a more fulminant symptomatic presentation, diagnosis of chronic meningitis is a challenge for clinicians. Cryptococcosis, coccidioidomycosis, and tuberculosis (TB) show neuroimaging features similar to those of hydrocephalus [3]. Inflammatory processes caused by these conditions may extend to the basal cisterns and may impede cerebrospinal fluid (CSF) circulation and absorption, leading to communicating hydrocephalus [4]. Cryptococcal meningitis (CM) and tuberculous meningitis can be fatal without proper treatment, and misdiagnosis of the underlying condition may result in increased disease severity. This case report presents a patient who complained of symptoms of NPH, mainly cognitive decline and gait disturbance, for several months and who was initially misdiagnosed and later found to have CM with cryptococcoma.
This case report was approved from the Institutional Review Board (IRB) of CHA Medical Center (No.2023-07-042). The need to obtain patient's written informed consent was waived by IRB.
Case Report
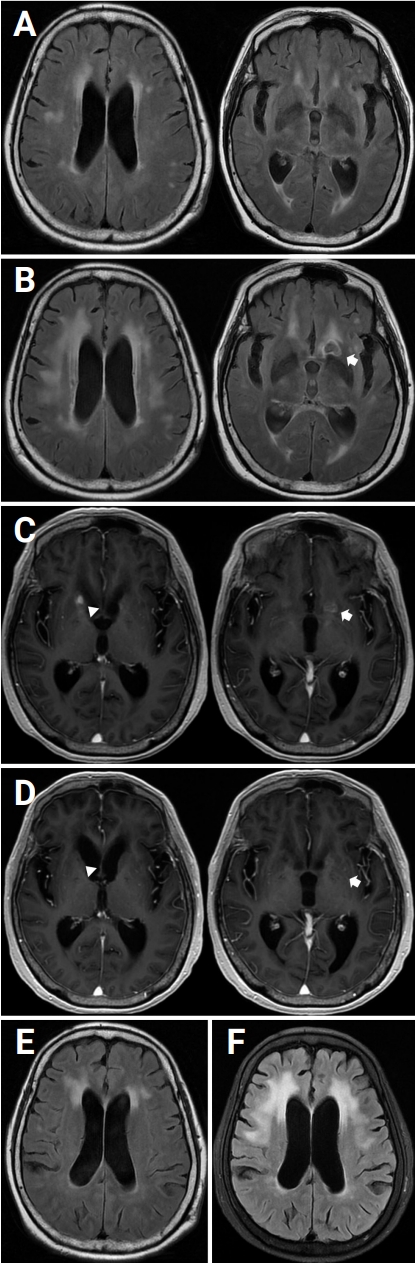
A 76-year-old female visited the outpatient clinic with a chief complaint of gait disturbance with recurrent falls and cognitive decline beginning 1 month prior. She had no previous medical history except for mild lumbar intervertebral disc herniation. During walking, she experienced difficulties making turns, requiring multiple small, shuffling steps. Brain magnetic resonance imaging (MRI) revealed ventriculomegaly without intraventricular obstructive lesions with relatively crowded sulci in the vertex, indicating a communicating hydrocephalus, such as that seen in patients with NPH (Figure 1A). She was supposed to be evaluated for dementia but was lost to follow-up. One month later, remarkable cognitive decline was witnessed by her family. After 4 months, she was unable to walk without assistance due to severely impaired balance with instability and a festinating gait with a tendency for small, rapid steps, making it challenging to stop or change direction. Based on her family’s observations, she was estimated to have a score of 4 or higher on the modified Rankin Scale. She visited another primary outpatient clinic for evaluation of NPH and underwent lumbar puncture and repeat MRI. Initial CSF analysis at the primary clinic revealed a white blood cell (WBC) count of 36 cells/mm3 with 94.5% lymphocytes. A new lesion that resembled an encapsulated abscess was found in the left caudate head on brain MRI with enhancement (Figure 1B). The patient was transferred to our hospital to review the findings. The Korean-Mini Mental State Examination (K-MMSE) was performed to assess cognitive decline, and the results were K-MMSE score of 19, Global Deterioration Scale score of 4, and Clinical Dementia Rating score of 2. Repeat CSF analysis after cisternography showed an open pressure of 9.5-cm CSF, a WBC count of 63 cells/mm3 with 90% lymphocytes, protein of 167 mg/dL, and glucose of 49 mg/dL (serum glucose, 227 mg/dL). The cultures for fungi, acid-fast bacteria (AFB) and other microorganisms, AFB staining, India ink staining, KOH mount, and TB polymerase chain reaction (PCR) were negative, whereas the cryptococcal antigen latex agglutination was positive (titer 1:4). The final diagnosis was chronic CM with cryptococcoma, and intravenous (IV) amphotericin B of 0.7 mg/kg/day with oral fluconazole of 800 mg/day was administrated. On the 5th day after starting antifungal therapy, the patient experienced nausea and vomiting, suggesting an increase in ICP. CSF drainage of 50 mL was performed via a lumbar puncture to manage ICP, and the patient’s condition improved. CSF analysis showed an open pressure of 15-cm CSF, a WBC count of 13 cells/mm3 with 95% lymphocytes, and a protein level of 130 mg/dL; however, the cryptococcal antigen was still positive (titer 1:4). The patient was discharged after 2 weeks of IV amphotericin B with a combination of oral fluconazole for induction therapy and was prescribed oral fluconazole of 800 mg/day for another month for consolidation therapy.
Serial brain MRIs
(A) Ventriculomegaly without intraventricular obstructive lesion with relative crowding of sulci in the vertex, indicating communicating hydrocephalus. (B) Encapsulated abscess-like lesion in the left caudate head (arrow). (C) Newly noted enhancement in the right caudate head (arrowhead) and decreased size of a presumed similar lesion in the left caudate head (arrow). (D) Decreased enhancement in the left caudate head (arrow) and resolution of enhancement in the right caudate head (arrowhead). (E) Mild to moderate white matter ischemic changes and diffuse mild brain atrophy on initial brain MRI. (F) Severe T2 hyperintensities in bilateral cerebral white matter (extending to anterior temporal white matter) and diffuse moderate brain atrophy.
MRI, magnetic resonance imaging.
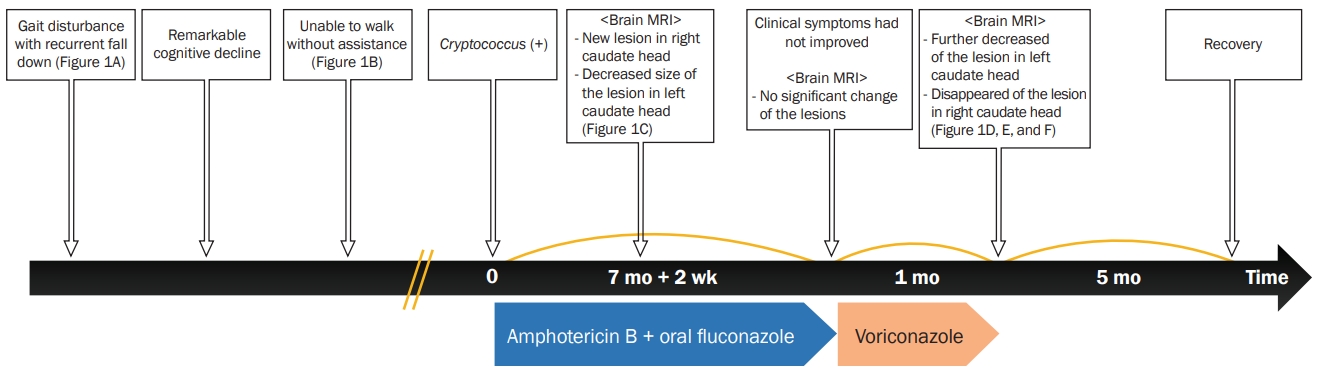
Two and one-half months after the patient started antifungal therapy, a follow-up brain MRI (Figure 1C) with contrast revealed new T1 high signal intensity lesions with enhancement in the right caudate head and decreased size of the presumed similar lesion in the left caudate head. Ventriculomegaly persisted without change on brain MRI. After continued maintenance therapy with oral fluconazole for another 5 months, however, no significant change of peripheral enhancement in the left caudate head was observed on brain MRI with enhancement, and clinical symptoms had not improved. We prescribed voriconazole (400 mg/day) for another month. On the first follow-up (1 month after starting oral voriconazole), a brain MRI with enhancement showed further decreased enhancement in the left caudate head and resolution of enhancement in the right caudate head (Figure 1D). Although hydrocephalus with subcortical white matter changes did not improve significantly (Figure 1E and F), the clinical symptoms steadily improved, and the patient recovered enough to walk independently at 3 months after tapering the antifungal therapy. Figure 2 summarizes the clinical and treatment course.
Discussion
NPH is difficult to diagnose because hydrocephalus has many causes, and ventriculomegaly is common in the elderly [1]. Thus, brain MRI and tests such as lumbar puncture with high-volume CSF removal or external CSF drainage that evaluate whether the symptoms can be improved are important. Additionally, when attempting lumbar puncture drainage, the CSF should be analyzed, and tests including cell counts, gram stains, microbial culture, PCR, and immunohistochemistry should be performed.
Our patient was initially misdiagnosed with NPH based solely on brain MRI and clinical features. A more in-depth evaluation revealed CM masquerading as NPH. However, the initial lack of detailed evaluation allowed aggravation of symptoms that resulted in irreversible brain injury.
The clinical features of CM include headache, fever, malaise, and confusion, and meningitis is one of the most common forms of central nervous system cryptococcosis [5]. A very rare manifestation of cerebral cryptococcosis is an intracranial mass lesion, defined as cryptoccoma, which shows ring-shaped enhancements in parts of the brain including the basal ganglia, cerebellum, or parietal lobe, similar to the findings in our patient [6]. A prospective study previously reported invasion of the brain in 44% of non-immunosuppressed patients, and the mortality rate was greater in non-immunosuppressed patients than in those infected with human immunodeficiency virus or immunosuppressed patients [7]. In addition, ICP can be elevated as a complication of CM, and an intracranial space-occupying lesion can cause severe neurologic deficits and poor prognosis [5]. In a previous case report of a patient with CM masquerading as NPH [8], the patient did not recover due to delayed diagnosis of CM, as confounding agents indicated NPH. Therefore, if there is a sign of communicating hydrocephalus in brain images with suspected NPH, it is necessary to confirm the improvement of clinical symptoms through lumbar puncture drainage, and differential diagnoses of etiologies that can cause hydrocephalus should be addressed via CSF analysis during lumbar puncture drainage. On the other hand, since ventriculomegaly was observed on brain MRI prior to diagnosis of CM in this case, it is possible that the patient had initial NPH symptoms and later developed CM, which exacerbated the symptoms.
In CM, uncorrected high ICP is the primary reason for failure of treatment [9]. Therefore, effective control of high ICP is important for successful treatment. The Infectious Disease Society of America (IDSA) has recommended repeated lumbar punctures as the first attempt and ventriculoperitoneal (VP) shunting as the second [10]. In our case, the patient underwent lumbar puncture twice, which improved her clinical symptoms. During hospitalization, there were considerations for VP shunting, which could have minimized neuronal injury such as subcortical white matter changes if it had been performed earlier. However, the neurosurgeon did not consider the patient a good candidate for VP shunt due to age and two previous lumbar puncture drainages.
According to current IDSA guidelines, amphotericin B should be used in combination with fluconazole (≥800 mg/day) when flucytosine is not available for induction therapy for 2 weeks. Long-term fluconazole therapy for consolidation (4 or more weeks) and maintenance therapy (6 to 12 months) is recommended because of the high relapse rates when therapy is discontinued [6]. However, the side effects of amphotericin B have resulted in its decreased use, and its combination with fluconazole does not reduce the mortality associated with CM [11]. Voriconazole is a relatively new broad-spectrum antifungal agent with good blood-brain barrier penetration. Recently, some case reports have shown that voriconazole could be used as an alternative treatment for patients with CM whose standardized treatment failed. A retrospective study demonstrated that patients receiving amphotericin B plus flucytosine combined with voriconazole showed rapid improvement in clinical manifestations, decreased CSF opening pressure, and lower blood potassium loss than those receiving amphotericin B plus flucytosine combined with fluconazole [12,13]. In our case, as the patient’s signs on brain MRI did not clearly improve, voriconazole was prescribed for 1 month, and the patient eventually recovered independent walking. Thus, voriconazole could be administered as an alternative antifungal agent to patients in whom conventional treatment fails.
A limitation of this study was that confirmation of the cryptococcal antigen titer through a CSF study would have allowed assessment of continuous cryptococcal production and to obtain a more reliable result based on the high sensitivity of the cryptococcal antigen test [14]. However, as the patient was continuously followed up in an outpatient clinic to assess changes in symptoms, a CSF follow-up study could not be performed, and the cryptococcal antigen titer test could not be performed.
Also, although it did not occur in this case, postinfectious inflammatory response syndrome (PIRS), characterized by recurrence or worsening of symptoms that often occur when treating CM, should be considered. PIRS is thought to result from an abnormal immune response triggered by clearance of the infectious agent. Diagnosis is based on clinical presentation and may involve increased CSF cell count and inflammatory markers. Treatment focuses on controlling inflammation with corticosteroids while addressing the underlying cause by optimizing antifungal therapy. PIRS requires prompt recognition to prevent long-term neurological complications and careful management balancing anti-inflammatory measures with the need for continued infection control [15]. Therefore, when the condition of a patient being treated for CM worsens, PIRS should be considered.
When diagnosing patients with NPH who present with communicating hydrocephalus in brain images, the cause of infection/inflammation involving the meninges should be considered. In addition, differential diagnoses of specific infectious etiologies of chronic meningitis should be addressed. If the clinical course is first monitored in patients with NPH without immediate CSF tapping, it is important to closely observe repeated follow-up brain images.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Shin JW; Data curation and Writing-original draft: Seo T; Writing-Review and editing: all authors