|
 |
| Encephalitis > Volume 3(4); 2023 > Article |
|
Abstract
Pneumocephalus refers to a pathologic collection of gas within the cranial cavity and is mostly caused by head trauma and neurosurgical procedures. Spontaneous nontraumatic pneumocephalus is a very rare condition. We herein report an unusual case of community-acquired bacterial meningitis with a combination of acute otitis media, Enterobacter cloacae, and nontraumatic pneumocephalus. A 75-year-old woman presented with fever, mental change, and neck stiffness. Brain imaging demonstrated pneumocephalus and fluid collection in the left mastoid air cells. E. cloacae was isolated from both blood and otorrhea cultures, and the patient was successfully treated with intravenous ceftazidime for 3 weeks. Although E. cloacae is a very rare cause of community-acquired bacterial meningitis in adults, it should be considered as a possible pathogen in otogenic meningitis complicated with pneumocephalus.
Pneumocephalus refers to a pathologic collection of gas within the cranial cavity and is mostly caused by head trauma and neurosurgery. Spontaneous nontraumatic pneumocephalus is a rare condition, and its etiologies include bone defect, congenital malformation, tumor, and infection [1]. Although very rare, bacterial meningitis has also been reported as an infectious cause of spontaneous pneumocephalus [2]. In community-acquired bacterial meningitis, bacteria invade the meninges by hematogenous spread or direct intracranial extension of adjacent infection [3]. In particular, bacterial meningitis can be caused by acute or chronic middle ear infection, which is referred to as otogenic or otitic meningitis. Because otogenic bacterial meningitis has a poor prognosis, prompt diagnosis and appropriate treatment are essential [4]. We report a case of community-acquired bacterial meningitis with an extremely rare combination of acute otitis media, Enterobacter cloacae, and nontraumatic pneumocephalus.
The study was approved by the Institutional Review Board (IRB) of Kangbuk Samsung Hospital (No. 2023-09-010) and the IRB waived the requirement to obtain informed consent.
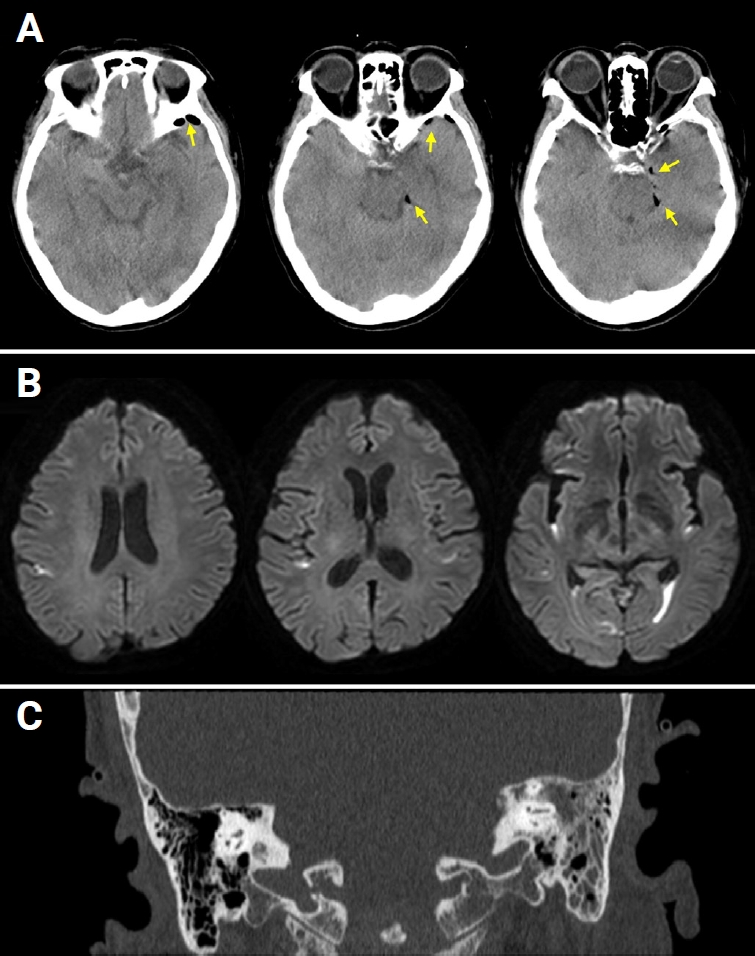
A 75-year-old woman visited the emergency room with a fever and mental change. She had complained of itching in her left ear for the past 2 weeks. One day prior to presentation, she experienced a severe headache on her left side. On the day of the visit, she was found unconscious in her bedroom with no evidence of head trauma. Her past medical history included current medication for hypertension and diabetes mellitus but no immunocompromised conditions. Initial vital signs were as follows: blood pressure, 176/96 mmHg; heart rate, 112 beats/min; respiration, 16 breaths/min; and body temperature, 39.9 ¬įC. On neurologic examination, her mental status was drowsy with confusion and irritability, and prominent neck stiffness was noted. Cerebrospinal fluid (CSF) findings were opening pressure of 18.5 cmH2O, white blood cell (WBC) count of 1,466 cells/¬ĶL (neutrophils, 99%), protein of 560 mg/dL, and glucose of 89 mg/dL (serum glucose, 235 mg/dL; ratio, 37.9%), suggesting bacterial meningitis. Brain computed tomography (CT) revealed a small amount of pneumocephalus along the left temporal lobe with no evidence of temporal bone fracture or skull defect (Figure 1A). Brain diffusion-weighted magnetic resonance imaging (MRI) showed hyperintensities in the dependent portion of the bilateral lateral ventricles and cerebral sulci, indicating ventricular empyema (Figure 1B). In addition, fluid collection in the left middle ear cavity and mastoid air cells was observed on brain MRI and CT (Figure 1C), and otoscopic examination confirmed acute otitis media on the left.
Empirical antibiotics were started to address the causative microorganisms of otogenic bacterial meningitis and ventricular empyema; vancomycin 1 g every 12 hours, ceftazidime 2 g every 8 hours, and metronidazole 500 mg every 8 hours. Adjuvant dexamethasone therapy was started before the first dose of antibiotic treatment. In addition, ventilation tube insertion and wide myringotomy were performed for acute otitis media. Her mental status rapidly improved within a few days. E. cloacae was isolated from both blood and otorrhea cultures, although CSF cultures were negative. E. cloacae isolates were susceptible to third-generation cephalosporins including cefotaxime and ceftazidime. Under the diagnosis of otogenic E. cloacae meningitis, intravenous ceftazidime was continued for 3 weeks, and the patient recovered without any neurological deficit. On day 21, CSF profiles were normalized to opening pressure of 9.5 cmH2O, WBC of 6 cells/¬ĶL (lymphocytes, 99%), protein of 32.6 mg/dL, and glucose of 80 mg/dL (serum glucose, 134 mg/dL; ratio, 59.7%), and pneumocephalus and ventricular empyema had resolved upon follow-up MRI.
Infection is a relatively common cause of spontaneous nontraumatic pneumocephalus. Among 140 cases of spontaneous nontraumatic pneumocephalus, 38 were associated with infections such as sinusitis, meningitis, subdural empyema, and osteomyelitis [1]. Among infectious conditions, meningitis accounted for 47.4% (18 cases), and the causative bacteria include Streptococcus pneumoniae, Bacteroides fragilis, Escherichia coli, Clostridium perfringens, Clostridium septicum, and Klebsiella pneumoniae. However, there have been only two reported cases of pneumocephalus secondary to E. cloacae meningitis; one is an adult case with ventilator-associated pneumonia and nosocomial meningitis [5], and the other case is neonatal meningitis in a 5-day-old infant [6]. To the best of our knowledge, our report is the first case of community-acquired E. cloacae meningitis in an adult that was complicated with nontraumatic pneumocephalus.
There are two major pathomechanisms by which bacterial infection causes pneumocephalus. The first is gas formation within the cranial cavity by the infected bacteria, and the second is erosive bone defect allowing air to enter the cranial cavity [1]. In our case, acute otitis media was the infection source of bacterial meningitis, but there was no evidence of bone defects or fistulous communication with the middle ear cavity. Therefore, it is most likely that acute otitis media spread intracranially to cause bacterial meningitis, and gas-forming bacteria caused pneumocephalus. Gas-forming infections are commonly caused by anaerobic bacteria, such as C. perfringens and B. fragilis, but facultatively anaerobic bacterial infections, including E. coli, K. pneumoniae, various streptococci, and E. cloacae, can also produce gas [7].
Another point in this case is that E. cloacae was the causative bacteria of otogenic meningitis. Bacterial meningitis is a well-known intracranial complication of otitis media. The common pathway by which middle ear infections spread intracranially is osteothrombophlebitis involving the venules of the adjacent mastoid and temporal bone [8]. Although both direct invasion and hematogenous spread were possible routes of infection, the presence of pneumocephalus limited to the left temporal lobe supported direct invasion from left otitis media. It is assumed that E. cloacae invaded the bloodstream during the intracranial spread and resulted in bacteremia. In otogenic meningitis, S. pneumoniae is the leading causative pathogen, and others include Staphylococcus aureus, Haemophilus influenzae, Pseudomonas aeruginosa, and anaerobes [9]. Therefore, empirical antibiotics for otogenic bacterial meningitis should be modified to include vancomycin, antipseudomonal cephalosporins such as ceftazidime or cefepime, and anti-anaerobic antibiotics such as metronidazole [10]. However, Enterobacter species are a very rare cause of otitis media [11], and to the best of our knowledge, otogenic meningitis caused by E. cloacae has never before been reported.
In the diagnosis of bacterial meningitis, blood cultures are valuable to identify the causative organisms and their susceptibility to antibiotics, especially when CSF cultures are negative. Blood cultures have been reported to be positive in approximately 60% of adults with bacterial meningitis [4]. In our case, although the CSF culture was negative, E. cloacae was isolated from both blood and otorrhea cultures, indicating it as the causative organism.
Enterobacter is a gram-negative, facultatively anaerobic, rod-shaped bacteria, among which E. cloacae is the most common species isolated in clinical settings [12]. Enterobacter is predominantly associated with nosocomial infections, particularly prevalent among critically ill patients receiving mechanical ventilation, rather than community-acquired infections. Enterobacter is an uncommon pathogen in adult bacterial meningitis; in a single-center study, Enterobacter infection accounted for 4.5% of culture-proven bacterial meningitis in adults [13]. Notably, nine of 10 patients with Enterobacter meningitis were associated with neurosurgery or head trauma. In another study, Enterobacter species were the third most common cause of healthcare-related meningitis, but they were not isolated in community-acquired meningitis [14]. In other words, Enterobacter is a very rare cause of community-acquired bacterial meningitis. In our case, E. cloacae isolates were susceptible to third-generation cephalosporins, and meningitis was successfully treated with ceftazidime. However, because the resistance of E. cloacae to third-generation cephalosporins has gradually increased, fourth-generation cephalosporins or carbapenems should be considered if clinically indicated [15].
In summary, this is the first case report of otogenic meningitis caused by E. cloacae infection and complicated with nontraumatic pneumocephalus. Although E. cloacae is a very rare cause of community-acquired bacterial meningitis in adults, it should be considered as a possible pathogen in otogenic meningitis complicated with pneumocephalus.
Figure 1.
Neuroimaging findings
(A) Brain computed tomography (CT) showed a small amount of pneumocephalus along the anterior and medial borders of the left temporal lobe (arrows). (B) Brain magnetic resonance imaging demonstrated diffusion-restricted signals in the dependent portion of the bilateral lateral ventricles and cerebral sulci. (C) Fluid collection in the left mastoid air cells was observed on temporal bone CT.
References
1. ŇömiŇāowska K, Sznajder-Stacha K, KocyŇāowski D, et al. Pneumocephalus as a rare complication: a systematic review plus clinical vignette. Neurol Neurochir Pol 2021;55:253‚Äď268.


2. Maliwan N. ‚ÄúSpontaneous‚ÄĚ pneumocephalus associated with mixed aerobic-anaerobic bacterial meningitis. J Infect Dis 1985;152:847‚Äď848.


3. Hoffman O, Weber RJ. Pathophysiology and treatment of bacterial meningitis. Ther Adv Neurol Disord 2009;2:1‚Äď7.



4. van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004;351:1849‚Äď1859.


5. Kuo CC, Wang JY, Chien JY, et al. Nontraumatic pneumocephalus due to nosocomial Enterobacter cloacae infection. Diagn Microbiol Infect Dis 2010;66:108‚Äď110.


6. Sedaghatian MR, Ramachandran P, Rashid N. Diffuse pneumocephalus caused by neonatal Enterobacter cloacae meningitis. Arch Dis Child Fetal Neonatal Ed 2004;89:F324.



7. Pasternack MS, Swartz MN. 96 - Myositis and myonecrosis. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett‚Äôs principles and practice of infectious diseases. 8th ed. W.B. Saunders; 2015. p. 1216‚Äď1225.e2.

8. Dubey SP, Larawin V, Molumi CP. Intracranial spread of chronic middle ear suppuration. Am J Otolaryngol 2010;31:73‚Äď77.


9. Barry B, Delattre J, Vi√© F, Bedos JP, G√©hanno P. Otogenic intracranial infections in adults. Laryngoscope 1999;109:483‚Äď487.


11. Kim SH, Jeon EJ, Hong SM, et al. Bacterial species and antibiotic sensitivity in Korean patients diagnosed with acute otitis media and otitis media with effusion. J Korean Med Sci 2017;32:672‚Äď678.




12. Davin-Regli A, Lavigne JP, Pag√®s JM. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin Microbiol Rev 2019;32:e00002‚Äď19.




13. Huang CR, Lu CH, Chang WN. Adult Enterobacter meningitis: a high incidence of coinfection with other pathogens and frequent association with neurosurgical procedures. Infection 2001;29:75‚Äď79.



- TOOLS
-
METRICS

-
- 0 Crossref
- 0
- 913 View
- 26 Download
- ORCID iDs
-
Jun-Sang Sunwoo

https://orcid.org/0000-0001-8834-0568 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print