Chronic social stress in early life can predispose mice to antisocial maltreating behavior
Article information
Abstract
Purpose
In our previous study, we developed an assay system to evaluate antisocial maltreating behavior of conspecific mice using a perpetrator–victim paradigm. We also generated a mouse model for the maltreating behavior by mimicking child maltreatment or abuse. Here, we further investigate the antisocial behavior using anti-aggressive and antipsychotic drugs.
Methods
Model mice sequentially subjected to maternal separation (MS), social defeat (SD), and social isolation (SI) in that order (MS/SD/SI model) were subjected to a maltreating behavioral task. The MS/SD/SI mice were treated with oxytocin (OXY), clozapine (CLZ), haloperidol (HAL), and 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). Western blotting and enzyme-linked immunosorbent assay were used for protein analysis.
Results
A substantial portion of the MS/SD/SI model mice (46% of males and 40% of females) showed a higher number of nose pokes than the control. OXY or 8-OH-DPAT treatment reduced the high number of nose pokes by the MS/SD/SI mice, whereas HAL increased it. CLZ did not affect the number of nose pokes by the MS/SD/SI mice. Interestingly, although the OXY level in the MS/SD/SI mice was similar to that in the control, the amount of OXY receptor was lower in the MS/SD/SI mice. The amount of 5-HT1A receptor was also decreased in the MS/SD/SI mice.
Conclusion
Chronic social stress in childhood might predispose a mouse to antisocial behavior. Our maltreating behavior assay system, including the MS/SD/SI model, is a good animal system for research on and drug screening for brain disorders associated with antisocial or psychotic behavior.
Introduction
Chronic environmental stress, including social stress, is known to induce inflammation in the brain, which is closely related to the psychiatric or psychosocial behaviors shown in brain disorders such as schizophrenia, dementia, encephalitis, autism, and psychopathy [1-27]. In humans, early-life stress (child and adolescent abuse and neglect) can induce structural or functional alterations in the brain, and chronically, repetitively abused or maltreated children often develop antisocial behavior, such as aggressive or violent and sadistic behaviors, in adulthood. In addition, humans exposed to maltreatment as children are at increased risk for the development of conduct disorders, personality disorders, major depression, posttraumatic stress disorder, schizophrenia, and anxiety disorders [28-30]. Empathy deficit is closely related to antisocial and maltreating behaviors [31-36], and recent animal studies revealed that emotional contagion or empathy and prosocial behavior occur in rodents [37-40].
Research in animals and humans has revealed that early-life stress affects brain development and social behavior in adulthood [7,28,30,41-45]. In our previous studies, we reported a mouse model generated by a sequential process of early-life social stress imitating child abuse or maltreatment: maternal separation (MS), social defeat (SD), and social isolation (SI). We found that animals subjected to the MS/SD/SI process displayed antisocial maltreating behavior in our perpetrator–victim system [44,45]. In this study, we further characterize the antisocial maltreating behavior of MS/SD/SI model mice using substances that affect social or antisocial behavior: oxytocin (OXY), clozapine (CLZ), haloperidol (HAL), and 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT).
Methods
Animals
Animals were maintained with free access to food and water under a 12-hour light/dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital (No. 14-0253-S1A1), and all animals were maintained in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (No. 001169) in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition (National Research Council 2011). All efforts were made to minimize suffering.
MS/SD/SI model animals were generated as described previously [44,45]. To mimic early-life social stresses, pups sequentially underwent MS, SD, and SI procedures. The MS procedure started from P4 to P18. During this period, litters were separated from their dams and placed in a new cage for 3 hours per day, and then they were returned to their home cages. SD, a second social stress paradigm, proceeded for those mice from P21 to P34. During this period, the mice were singly placed as intruders into the cages of singly housed aggressor C57BL/6 mice (territorial residents, 8- to 15-week-old male) for 10 minutes per day for 10 days between P21 and P34. At the beginning of the SD procedure (P21), the mice were separated from their littermates and housed singly (SI) until all experiments were finished. The maltreating behavioral task was started at the age of 9 weeks. The MS/SD model was generated using only MS and SD without SI, and the SI model was generated using only SI, without MS and SD. All experiments were conducted on male C57BL/6 mice unless otherwise stated.
Apparatus and procedure for the maltreating behavior task
The apparatus and procedure for the maltreating behavior task were described previously [45]. The apparatus consists of two chambers with a stainless-steel grid rod floor (5-mm diameter rods, spaced 0.7 cm apart) partitioned by a transparent Plexiglas divider: the victim compartment is 10 × 6 × 21 cm, and the perpetrator compartment is 20 × 10 × 21 cm (Figure 1A). The grid floor in the victim compartment is exposed, whereas an opaque acrylic plate (20 × 10 × 0.3 cm) covers the grid floor in the perpetrator compartment to keep the perpetrator mouse off the electric shocks. The perpetrator compartment contains a nose poke near the transparent divider. The nose-poke module includes an infrared control, source, and detector. Whenever the beam of the nose poke is broken by the entry of the perpetrator’s nose, a 2-second foot shock (1 mA) is delivered to the mouse in the victim compartment through the grid floor via a computer-controlled animal shocker. The number of nose pokes (equal to the number of beam breaks and electric shocks delivered) is recorded in real-time and analyzed every 10 minutes to assess level of maltreating behavior. All modules and paradigms were programmed using the MED-SYST-8 interface and software package (Med Associates Inc.).

Maltreating behavior task
(A) Photo of the experimental device for measuring maltreating behavior. (B) The number of nose pokes by each male MS/SD/SI model mouse (n = 46) during 120 minutes. The mice were divided into two groups: MS/SD/SI-High and -Low. (C, D) The MS/SD/SI-High (n = 25), but not the MS/SD/SI-Low group (n = 21), showed an increased number of nose pokes compared with the control mice (n = 17). (E, F) Female MS/SD/SI mice were also divided into two groups: MS/SD/SI-High (n = 12) and -Low (n = 8). **p < 0.01, two-way analysis of variance (ANOVA). ##p < 0.01, one-way ANOVA.
MS, maternal separation; SD, social defeat; SI, social isolation.
For the maltreating behavior task, the mice (perpetrator and victim) are individually placed in their apparatus chambers for 10 minutes, during which the hole of the nose poke is covered with a panel that is the same as that used for the walls of the chamber. When the screen panel is removed after the 10-minute habituation period, the perpetrator mouse can easily find the nose poke. The first experiment was run for 120 minutes. Electric shocks were delivered to the victim mouse only at the moment when the perpetrator mouse put its nose into the hole of nose poke. Repetitive maltreating behavior tasks were performed at intervals of 5 to 7 days, and those experiments lasted for 30 or 40 minutes.
Drugs and repetitive maltreating behavior
OXY, CLZ, HAL, and 8-OH-DPAT were purchased from Sigma Aldrich. The doses of the drugs were chosen on the basis of previous studies showing that OXY (0.5 mg/kg), CLZ (0.5 mg/kg), HAL (0.05 mg/kg), and 8-OH-DPAT (0.1 mg/kg) did not produce any effects on locomotion [46-49]. Some perpetrator mice in the MS/SD/SI-High group, which showed a high number of nose pokes, were used repeatedly for drug treatment experiments. They were intraperitoneally treated with OXY, CLZ, HAL, or 8-OH-DPAT 20 minutes before being placed in the apparatus chamber. Each drug, including the vehicle control, was randomly administered to the same mice at intervals of 5 to 7 days, and the maltreating behavior task for each drug was run for 30 minutes. The saline vehicle was administered twice during the drug-experiment period, and the average number of nose pokes from those tests was used as the control.
Blood and tissue sampling
Mouse anesthesia was induced by an intraperitoneal injection of ketamine hydrochloride (100 mg/kg) and xylazine (10 mg/kg). Within an hour after the last maltreating behavior test, all mice were sacrificed, and blood samples were obtained by heart puncture (heparin was used as an anticoagulant). The samples were centrifuged at 3,000 g and 4 ℃ for 20 minutes, and plasma was stored at –70 ℃ until the enzyme-linked immunosorbent assay (ELISA). Brain tissues were also rapidly extracted within an hour after a maltreating behavior test and immediately frozen in liquid nitrogen.
Protein sample preparation
Total protein extracts were obtained from the whole brain. The frozen brains were weighed and suspended in 2 mL of lysis buffer (ice-cold phosphate-buffered saline solution, pH 7.2, supplemented with a protease inhibitor cocktail, 1-mmol phenylmethylsulfonylfluoride, 0.5% Triton X-100) per gram of tissue. Tissue was disrupted using sonication and then centrifuged at 13,000 g and 4 ℃ for 30 minutes. The total protein concentration in the supernatants was quantified using a bicinchoninic acid colorimetric assay kit (Pierce BCA protein assay kit, cat.23225; Thermo Fisher Scientific). The prepared protein sample was used for ELISA or western blotting.
Enzyme-linked immunosorbent assay analysis
The OXY levels in extracted blood plasma were measured using a competitive ELISA kit (ADI-900-153A, Enzo Life Sciences). Briefly, plasma samples were diluted 1:4 with assay buffer, loaded in duplicate into wells with serial diluted OXY standards, and left overnight at 4 ℃. The next day, the excess reagents were washed away, and the bound OXY phosphatase was incubated with a substrate. After a 1-hour incubation, the enzyme reaction was stopped, and the optical density at 405 nm was read using a VERSAmax microplate reader.
Western blotting analysis
The sample proteins (25 μg per lane) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and western blotting was performed with a primary antibody against OXY receptor (OXYR, 1:1,000; Abcam) or serotonin 1A receptor (5-HT1AR, 1:1,000; Abcam). Immunoreactive proteins were visualized with enhanced chemiluminescence reagents (Pierce, Thermo Fisher Scientific) and digitally scanned using a GS-700 scanner (Bio-Rad Laboratories). The optical density of each band was determined relative to the measured value of β-actin (Santa Cruz Biotechnology) bands using ImageJ software (National Institutes of Health).
Statistical analysis
All data are shown as the means ± standard error of the mean. An analysis of variance was used to conduct multiple comparisons of means. The Student t-test or Mann-Whitney U-test was performed to determine statistical differences between the two means. A p-value of <0.05 was considered to indicate statistical significance.
Results
Constant maltreating behavior with the maternal separation/social defeat/social isolation models
In the previous report, we showed that male MS/SD/SI model mice displayed an increased number of nose pokes in the maltreating behavior task [45]. After looking at the data for each mouse, we divided the male MS/SD/SI mice (n = 46) into two groups based on the highest total number of nose pokes in the control: MS/SD/SI-High and -Low groups (Figure 1B). Interestingly, a substantial portion of the MS/SD/SI model mice (MS/SD/SI-High, 21 of 46 mice) consistently showed a higher number of nose pokes throughout the behavioral experiment, and the others (MS/SD/SI-Low, 25 of 46 mice) displayed a number of nose pokes similar to the control (Figure 1C and D). Some female MS/SD/SI model mice also displayed a higher number of nose pokes than the control (Figure 1E), and the high and low groups showed a composition (MS/SD/SI-High, 12 of 20 mice; MS/SD/SI-Low, 8 of 20 mice) similar to the male MS/SD/SI mice (Figure 1E and F). Most of all, we found that both the male (n = 12, Figure 2A) and female MS/SD/SI-High groups (n = 8, Figure 2B) showed a constant and sustained high number of nose pokes without habituation or adaptation, even when they repeated the maltreating behavior task two or three times.

No adaptation or habituation was found in the maltreating behavior task
The male (A) and female (B) MS/SD/SI-High groups continued to exhibit a high number of nose pokes even in repeated experiments, showing persistent maltreating behavior.
MS, maternal separation; SD, social defeat; SI, social isolation.
Altered oxytocin signaling in the maternal separation/social defeat/social isolation models
OXY is known to affect social and empathic behavior [50,51], and we previously showed that OXY is involved in the maltreating behavior in our perpetrator–victim behavioral system [45]. To determine whether OXY can ameliorate maltreating behavior or improve social behavior, we measured the level of nose poking after we administered OXY to the MS/SD/SI-High group. The administration of OXY to MS/SD/SI-High group mice reduced the number of nose pokes compared with a vehicle injection (n = 19, 53.90% ± 7.05%; p < 0.01, Student t-test) (Figure 3A).
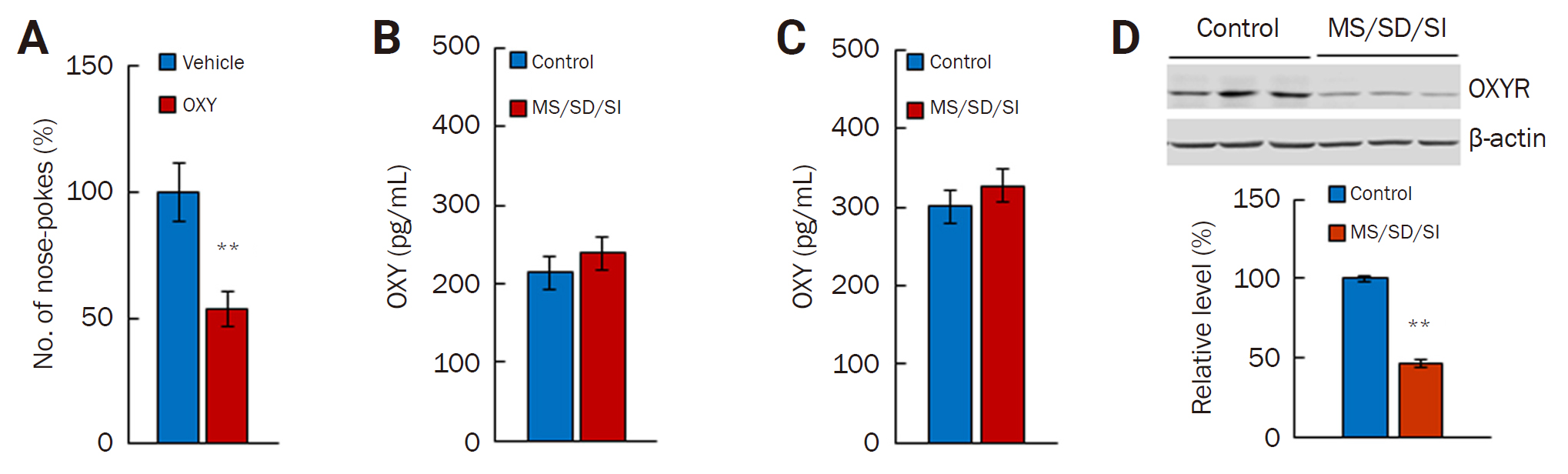
Alteration in OXY signaling in the brains of the MS/SD/SI model mice
(A) OXY treatment reduced the number of nose pokes by the MS/SD/SI-High group (n = 19) in a maltreating behavior task lasting 40 min. (B, C) The amount of OXY before (B) and after (C) the maltreating behavior task between the control and MS/SD/SI model mice. (D) The amount of OXYR differed significantly between the MS/SD/SI model mice (n = 7) and the control mice (n = 7). **p < 0.01, Student t-test.
OXY, oxytocin; MS, maternal separation; SD, social defeat; SI, social isolation; OXYR, OXY receptor.
We further investigated the levels of OXY and OXYR in MS/SD/SI model mice. First, we measured the level of OXY from the perpetrator serum. The amount of OXY did not differ between the control (n = 8) and the MS/SD/SI model mice before the maltreating behavior task (n = 8) (Figure 3B). In addition, although the amount of OXY increased in both the control (n = 14) and MS/SD/SI model mice (n = 10) after the maltreating behavior task, there was no difference between the groups (Figure 3C). Next, we measured the OXYR level in the brain. Notably, the MS/SD/SI model mice (n = 7) showed a lower level of OXYR than the control (n = 7, 46.20% ± 2.31%; p < 0.01, Student t-test) (Figure 3D). Taken together, these results indicate the involvement of OXY signaling in the maltreating behavior and the impairment of OXY signaling caused by a reduced OXYR level in the MS/SD/SI model animals.
Effects of antipsychotic drugs on the maltreating behavior of the maternal separation/social defeat/social isolation mice
We further investigated the maltreating behavior using drugs that can affect antisocial behaviors such as violence or aggressiveness: 8-OH-DPAT, an agonist of the 5-HT1AR, HAL, a typical butyrophenone-type antipsychotic antagonist of the dopamine (DA) D2 receptor, and CLZ, an atypical antipsychotic antagonist of the 5-HT and DA receptors. Administration of 8-OH-DPAT reduced the number of nose pokes by the MS/SD/SI-High-group mice (n = 20, 58.10% ± 5.90%; p < 0.01, Student t-test) (Figure 4A) compared with vehicle treatment. However, CLZ did not affect the number of nose pokes by the MS/SD/SI-High-group mice (n = 20). Interestingly, HAL increased rather than decreased the number of nose pokes by the high MS/SD/SI group mice, compared with vehicle treatment (n = 20, 149.42% ± 15.82%; p < 0.05, Student t-test) (Figure 4A).

Effects of 8-OH-DPAT, CLZ, and HAL on the maltreating behavior of MS/SD/SI mice
(A) 8-OH-DPAT treatment reduced the number of nose pokes by the MS/SD/SI-High group (n = 20) in a maltreating behavior task lasting 40 minutes. CLZ treatment did not reduce the number of nose pokes by the MS/SD/SI-High group, and HAL treatment increased the number of nose pokes. Comparison with vehicle **p < 0.01 and #p < 0.05, Student t-test. (B) Comparison of the amount of 5-HT1AR protein, shown by Western blotting. The model group (n = 7) seems to have a slightly lower amount of 5-HT1AR than the control (n = 7). *p < 0.05, Mann-Whitney U-test.
8-OH-DPAT, 8-hydroxy-2-(di-n-propylamino)tetralin; CLZ, clozapine; HAL haloperidol; MS, maternal separation; SD, social defeat; SI, social isolation; 5-HT1AR, serotonin 1A receptor.
Next, to determine whether there was a change in the expression of the 5-HT1AR, the main target molecule of 8-OH-DPAT, the amount of the 5-HT1AR protein was measured in the brains of MS/SD/SI mice. Notably, the MS/SD/SI model mice (n = 7) showed a lower level of 5-HT1AR than the control mice (n = 7, 76.17% ± 11.06%; p < 0.05, Mann-Whitney U-test) (Figure 4B).
Discussion
In our previous reports [44,45], we showed that MS/SD/SI model mice exhibited hyper-phenotypes of social interaction and offensive aggressiveness, hypo-phenotypes of predator fear and empathy-related behavior, and abnormality in maltreating behavior. In this study, we further investigated the MS/SD/SI model molecularly and pharmacologically to elucidate the neural mechanisms underlying the antisocial maltreating behavior.
We found that not all of the MS/SD/SI model mice exhibited deviant behavior in the maltreating behavior task. Only 46% of male and 40% of female MS/SD/SI mice used in the experiment showed a higher number of nose pokes than the control. Although we do not know how the model mice in the low group would respond to repeated trials, the high-group mice consistently showed high nose-poking behavior in repeated trials. This characteristic of the model enabled repeated experiments with several drugs in each individual. In consideration of possible adaptation, the repeated experiments were conducted for only 30 to 40 minutes. OXY and 8-OH-DPAT ameliorated the maltreating behavior of the MS/SD/SI model mice. Rather than changes in the release or amount of OXY and 5-HT, their receptor expression appears to change. However, HAL made the maltreating behavior worse. Although we used very low doses of HAL that did not affect the animals’ locomotion, the inhibition of dopamine signaling can affect learning and memory, which might explain why HAL exacerbated the maltreating behavior. How the DA system changed in the MS/SD/SI model requires further investigation. We do not believe that the maltreating behavior of the MS/SD/SI mice can be explained simply by changes in the neurotransmitter signaling system. Instead, we expect that changes in functional connectivity and synaptic structures in the brains of MS/SD/SI model mice are also involved in causing the maltreating behavior.
In conclusion, we demonstrate that cumulative chronic social stress in early life can predispose mice to antisocial maltreating or abusive behavior. Our maltreating behavior task might be an assay system for antisocial behavior related to empathy deficit. In addition, the MS/SD/SI process could be a good animal model of brain disorders associated with antisocial behavior, impaired empathy, and excessive boldness, such as psychopathy.
Notes
Conflicts of Interest
Daejong Jeon has been an Associate Editor of encephalitis since October 2020. Kon Chu and Sang Kun Lee also have served on the editorial board of encephalitis since October 2020. They were not involved in the review process of this original article. No other potential conflicts of interest relevant to this article was reported.
Author Contributions
Conceptualization, Funding acquisition, Resources, Project administration, Supervision: DJ, SKL, KC; Methodology, Investigation, Data curation, Formal analysis, Validation, Visualization: DJ, SK; Writing–original draft: DJ; Writing–review & editing: DJ, SKL, KC.
Acknowledgements
We thank Young-Sook Kim (Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea), Jiye Choi (Korea Advanced Institute of Science and Technology, Daejeon, Korea), and Ah Reum Yang (Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea) for their help in the protein assay and behavioral tests. This work was supported by a grant from Advanced Neural Technologies (0620182930).
