A case of leucine-rich glioma-inactivated 1 antibody encephalitis with schizophrenia-like symptoms as an initial clinical manifestation
Article information
Abstract
Leucine-rich glioma-inactivated 1 (LGI-1) antibody encephalitis is a type of limbic encephalitis characterized by faciobrachial dystonic seizure and short-term memory loss as initial clinical symptoms. We present a case initially misdiagnosed as schizophrenia and finally diagnosed as LGI-1 antibody encephalitis. A 41-year-old female presented to the neurology clinic with a 4-month history of anxiety and disoriented speech and a new onset headache. Her explanation of symptoms was unclear, and she was unable to answer questions properly. Her brain magnetic resonance imaging (MRI) showed no specific lesions. After 6 months, depersonalization, place disorientation and memory impairment were noted. Her symptoms continue to progress, experiencing visual/auditory hallucinations. She was diagnosed with schizophrenia and admitted to a closed psychiatric ward. In the hospital, she showed mild fever, and her memory loss worsened faster than her psychiatric symptoms, unlike in schizophrenia. Follow-up MRI scans showed a diffusely enlarged right hippocampus with a 2.5 × 1.3-cm mass lesion. Electroencephalogram showed rhythmic theta activities/interictal spikes in the right frontal lobe, for which she was treated with an antiepileptic drug. Cerebrospinal fluid analysis results showed pleocytosis. Based on this, autoimmune encephalitis was diagnosed, and steroid pulse treatment and immunoglobulin treatment were performed. Positivity for LGI-1 antibody was reported and finally led to diagnosis of LGI-1 antibody encephalitis. Clinical symptoms gradually improved, and the lesion had shrunk considerably on MRI performed 6 months after immunoglobulin treatment. She reports persistent amnesia for 6 months but has returned to her daily life under follow-up observation.
Introduction
Limbic encephalitis involves the medial temporal area and can present as abnormal behavior, altered mentality such as confusion and short-term memory loss, and convulsions. It often is difficult to determine the cause of the disease because the clinical symptoms and imaging results are similar for paraneoplastic syndrome and infectious causes. Efforts have been made to identify the antibodies in various types of autoimmune encephalitis including limbic encephalitis.
The protein leucine-rich glioma-inactivated 1 (LGI-1) is a voltage-operated potassium channel, dysfunction of which can cause limbic encephalitis. The authors experienced a patient with LGI-1 antibody-related limb encephalitis that was initially suspected of schizophrenia and report this case with a literature review.
Case Report
This report was approved by the Institutional Review Board at International St. Mary’s Hospital, Catholic Kwandong University (No. IS22RISI0064).
A 41-year-old female presented to the outpatient clinic with a 4-month history of anxiety, disoriented speech, and headache involving stinging pain from the back of the head to the right shoulder. Brain magnetic resonance imaging (MRI) at another hospital a few months prior showed no sign of active lesions. One month after imaging, she developed palpitation, dizziness, lethargy, and dyspnea and showed mild hyponatremia (serum Na 131 mEq/L).
The patient was a housewife with a 10-year career as an accountant. She had visited a psychiatric hospital for depression at 20 and 10 years prior but had no other medical history including autoimmune diseases, such as thromboembolic vasculitis. She had no smoking or drinking history and no family history of liver cancer, dementia, or other neurologic or psychiatric diseases.
At 6 weeks after her first visit, the characteristics of her headaches changed, involving depersonalization and memory loss, as well as impaired spatial memory and place orientation. She claimed to recognize nothing in her house, and her husband reported poor activity of daily living (ADL) including poor hygiene and poor management of housework, place disorientation such as wandering, profoundly disoriented speech, and visual hallucination. No specific findings were observed on brain computed tomography (CT).
After 1 week, with a chief complaint of recent memory loss for 4 days, she was referred to the department of neurology for transient global amnesia and special screening for other viral diseases. Her cognitive function was below normal (Korean version of Mini-Mental Status Examination [K-MMSE] was 24/30, significant time disorientation, place disorientation, impaired concentration and calculation, and impaired recall). She presented behavioral psychiatric symptoms like visual and auditory hallucinations, agitation, and irritability. The cerebrospinal fluid (CSF) results were negative, and there were no specific findings in other laboratory tests, electroencephalogram (EEG), and brain CT except mild hyponatremia (serum Na 132 mEq/L).
One week after discharge against medical advice, this patient was readmitted to the department of psychiatry for worsened disorganized speech and hallucinations. Treatment with the antipsychotics risperidone, quetiapine, and olanzapine and antianxiety drugs including lorazepam, clonazepam, and diazepam for 1 month produced no symptom improvement. Mild hyponatremia (serum Na 132 mEq/L) persisted. Two months after her readmission, fever developed and a diffuse, enlarged right hippocampus with a 2.5 × 1.3-cm questionable mass lesion was shown on brain MRI (Figure 1). CSF analysis showed mild pleocytosis of 9/mm3 with normal levels of protein (micro total protein, 37.4 mg/dL) and glucose (63 mg/dL). EEG showed novel rhythmic theta activities/interictal spikes in the right frontal lobe, for which she was treated with the antiepileptic drug oxcarbazepine at a 900-mg dosage. Finally, 3 months after her readmission, the CSF antibody study showed positivity for LGI-1 antibody, based on which she was finally diagnosed with LGI-1 antibody encephalitis.
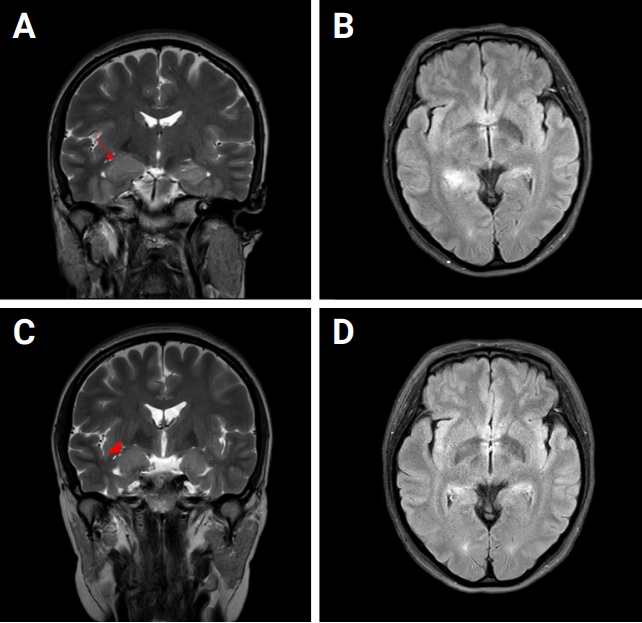
Brain MRI scans
(A) T2W-MRI coronal view showing the diffusely enlarged right hippocampus with a 2.5 × 1.3-cm suspicious mass lesion (arrow). (B) T2W-FLAIR image showing high signal intensities on the right posterior hippocampus and right pulvinar area of the thalamus. (C) T2W image obtained in the coronal view 6 months later, showing a mild edematous enlarged right-side hippocampus (arrowhead). (D) T2W-FLAIR image showing improvement.
MRI, magnetic resonance imaging; T2W, T2-weighted; FLAIR, fluid-attenuated inversion recovery.
EEG continued to show sharp waves in the frontal/temporal areas, her cognitive/memory impairment had not recovered, and nocturnal altered mentality and wandering/ myoclonic symptoms persisted. Therefore, the antiepileptic drugs (clonazepam 1 mg and perampanel 2 mg) were added to her treatment. After 5 days of steroid pulse therapy with prednisolone 60 mg per day and 10 mg tapering daily, her attention improved, but abnormal nighttime behavior persisted. She did not fully recover after steroid pulse therapy and was started on intravenous immunoglobulin (IVIG) treatment for 5 days. Intermittent confusion, abnormal nighttime behavior, and short-term memory loss improved dramatically after IVIG treatment. Her level of consciousness improved and the fever, headache, and nighttime hyperactivity resolved. Memory loss persisted for several weeks, but her psychological symptoms such as intermittent confusion and abnormal wandering had recovered enough for hospital discharge.
Two months after discharge, her memory had started to return, and ADLs had dramatically improved, allowing her to cook, drive, and take care of herself and her son. Nighttime wandering had resolved, but she reported vivid nightmares with a depressive mood. At 6 months after discharge, her cognitive function had recovered to mildly impaired status (K-MMSE was 25/30, impaired recall and time and place disorientation), and a follow-up brain MRI also showed improvement (Figure 1). The image was similar to that at the early stage of encephalitis, showing a diffuse mildly edematous enlarged right hippocampus without remarkable contrast enhancement. Her vivid nightmares and depressive mood persisted, and her memory impairment had not fully recovered but continued to improve.
In outpatient follow-up, she is treated with antiepileptic drugs to prevent seizure during recovery and antidepressants including aripiprazole for depressive mood. The antiepileptic drugs were changed to oxcarbazepine 600 mg and levetiracetam 500 mg after normalization of serum sodium level 10 months after the initial outpatient visit. After another 6 months after discharge, her neurologic/psychological status had improved, and the oxcarbazepine dosage was decreased to 300 mg and levetiracetam was discontinued. Clinical symptoms in chronological order showed in Figure 2.
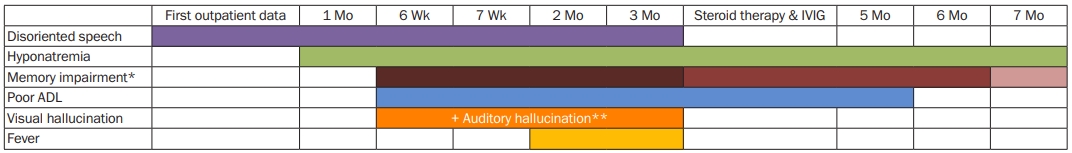
Clinical symptoms in chronological description
Disoriented speech, visual hallucination and fever has gone after steroid pulse therapy and IVIG treatment. Memory impairment ameliorated but not remissioned after the treatment. However, after the treatment, hyponatremia and poor ADL persisted for 4 months and 2 months each.
* For memory impairment, depth of color describes severity of symptoms.
** Auditory hallucination appeared at 7 weeks after the first outpatient visit.
ADL, activity of daily living; IVIG, Intravenous immunoglobulin.
Discussion
LGI-1 antibody encephalitis is autoimmune encephalitis involving autoantibodies of LGI-1 protein, which is secreted from the presynaptic terminal in the hippocampus or neocortex. This antibody triggers epileptiform activity in the hippocampus [1]. Faciobrachial dystonia is specifically related to LGI-1 protein expression in the basal ganglia [2]. Accordingly, an autoimmune disorder with antibodies against LGI-1 protein may cause limbic encephalitis. LGI-1 antibody encephalitis is predominant in males, accounting for 60%-70% of cases, and has been reported at many adult ages, with a median age of 60 years, in a range of 31-84 years [3]. The incidence is as rare as 0.83/million/year [4], although this might be an underestimation. Of cases, 5%-10% showed an association with thymoma [5].
According to Jang et al. [6], 75% of patients diagnosed with LGI-1 antibody encephalitis manifested psychiatric symptoms. Among the Korean case reports, only one reported psychiatric symptom as the initial manifestation of LGI-1 antibody encephalitis [7]. The presented case is the only one without a clinical seizure. At the early disease stage, there were no positive objective findings suggesting encephalitis, leading to an exclusionary diagnosis of schizophrenia. However, short-term memory loss was exacerbated with psychiatric treatments, and another encephalitis work up was performed. Since the disease progression here was different from previous case reports, this case is presented for its educational value to clinicians.
Seizure and cognitive disorder are the key symptoms of LGI-1 antibody encephalitis, and faciobrachial dystonic seizure (FBDS) involving the ipsilateral face and limb is typical [8]. FBDS (47%) and other subtle focal seizures (66%, autonomic or dyscognitive) often occur before onset of memory disturbance [4]. Tonic-clonic seizures (63%) and FBDS developed later and not as initial symptoms in 25% of patients [4,8]. Even though seizure is a key symptom of LGI-1 antibody encephalitis, the present patient experienced no clinical seizure including FBDS.
The most common presentation of cognitive impairment in LGI-1 antibody encephalitis is short-term memory impairment. Spatial disorientation, sleep disorders, and autonomic dysfunctions also can occur [8].
Although neurologic symptoms are more characteristic than psychotic symptoms in LGI-1 antibody encephalitis, hallucinations, emotional changes, and depressed mood can be present [9].
In LGI-1 antibody encephalitis, hyponatremia is a very common (70%) manifestation in serum study. The CSF test shows an abnormality in up to 25% of patients, often mild pleocytosis with elevated protein. In the EEG study, an abnormal pattern is shown in 50% of cases, 30% as epileptiform abnormality, and 20% as focal swelling. Abnormal brain MRI pattern is seen in up to 75% of patients, with 40% showing increased signal or swelling in the medial temporal lobes. A unilateral pattern is more common than a bilateral pattern [10]. Brain MRIs which were initially normal, had developed hippocampal atrophy or sclerosis later, suggesting that hippocampal inflammation was present but was not detectable by routine MRI in the acute phase [11]. Additionally, around 90% of LGI-1 antibody encephalitis patients show human leukocyte antigen (HLA)-DRB1*07:01 [12].
As first-line treatment, high-dose methylprednisolone, IVIG, and plasma exchange can be considered. Typical second-line therapy is mycophenolate, azathioprine, rituximab, and cyclophosphamide. Third-line or experimental therapies include bortezomib and tocilizumab. Since >90% of patients with LGI1-antibodies carry the HLA-DRB1*07:01 allele, T-cell-directed therapies might be an option in the future, as generation of antibody secreting cells and memory B cells occurs through engagement of HLA with T cell receptor [10,13,14]. Although ascertainment bias should be considered, 1/3 of patients fully recovered, 1/3 were functionally independent but unable to work, and 1/3 were severely disabled or dead within 2 years; relapse occurred in 20%–30% of cases and was associated with poor outcomes [10].
Most central nervous system (CNS) infections including meningitis or encephalitis are accompanied by infectious illness signs such as high fever, chills, and sudden neurological symptoms. Therefore, it is difficult to suspect CNS infection in patients without infectious illness. Here, we report a patient who was diagnosed with LGI-1 antibody encephalitis showing psychological symptoms similar to those in schizophrenia as an initial clinical presentation and absence of convulsive seizure. We present this case to demonstrate the unusual symptomatic progression of LGI-1 antibody encephalitis and to facilitate the diagnosis of LGI-1 antibody encephalitis with increased awareness.
We report a case of LGI-1 antibody encephalitis with schizophrenia-like symptoms as a rare initial clinical manifestation. The most common initial manifestation of LGI-1 antibody encephalitis is seizure, especially FBDS [8]. Interestingly, the presented patient experienced no convulsive seizure, although EEG intermittently showed epileptic waves and rare intermittent irregular mixed slow activities.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Project administration, Resources, Supervision, Validation: Kim H; Writing–original draft: Moon J; Writing–review & editing: Kim H.
