Myelin oligodendrocyte glycoprotein antibody-associated encephalitis after severe acute respiratory syndrome coronavirus 2 infection: a case report and retrospective case reviews
Article information
Abstract
Several cases of myelin oligodendrocyte glycoprotein (MOG) antibody-associated encephalitis have been reported after coronavirus disease 2019 (COVID-19). In this case, the patient presented with focal status epilepticus with impaired awareness, auditory hallucinations, and incoherent speech after COVID-19. Brain magnetic resonance imaging revealed no specific findings. Cerebrospinal fluid results showed pleocytosis and MOG antibody testing confirmed anti-MOG antibody with live cell-based fluorescence-activated cell sorting assay. The patient was diagnosed with MOG antibody-associated autoimmune encephalitis and treated with intravenous immunoglobulin, rituximab, and tocilizumab. This case occurred presumably due to auto-antibody production following COVID-19.
Introduction
Myelin oligodendrocyte glycoprotein (MOG) is a glycoprotein located on the oligodendrocyte surface that plays an important role in central nervous system (CNS) myelination. It is an immunogenic molecule with functions related to cytoskeletal stability and immune reaction. It is also presumed to play an important role in autoimmunity and cell-mediated immune responses and to be associated with autoimmune demyelinating disease [1,2]. Acute disseminated encephalomyelitis (ADEM)-like perivenous inflammatory demyelination with MOG-dominant myelin loss is a characteristic finding of MOG antibody-associated disease; also, CD4+ T-cell–dominant inflammatory cell infiltration was found in immunohistochemically-analyzed biopsied brain tissues. [3].
MOG antibodies are related to neuromyelitis optica spectrum disorder (NMOSD). However, due to differences in histopathology and inflammatory CNS lesions between AQP4-immunoglobulin G (IgG)–positive and MOG-IgG–positive patients, MOG-IgG disease (MOGAD) is believed to be a distinct disease entity from NMOSD [4].
There are several studies on the clinical features and imaging findings of MOGAD. As imaging findings of diseases related to MOG-IgG, various clinical magnetic resonance imaging (MRI) patterns of different diseases, such as optic neuritis, myelitis, encephalitis, ADEM, and brainstem/cerebral cortical encephalitis, may appear alone or in combination. In particular, ADEM-like patterns with diffuse signal changes in the cortical/subcortical white matter, deep white matter, and deep gray matter as seen on both T2-weighted and fluid-attenuated inversion recovery images are representative MRI findings of MOG encephalitis [5,6]. Considering that brain MRI abnormalities are reported in 45% to 77% of cases and spinal cord MRI abnormalities are reported in 50% of cases, there may be cases without abnormal MRI findings [4]. As a result of the retrospective analysis of clinical characteristics of 40 MOG antibody-associated autoimmune encephalitis (MOGAE) patients based on suspected clinical autoimmune encephalitis patient data in a recently published single-center prospective cohort, cases could be categorized into the following three groups: cortical encephalitis, limbic encephalitis, and ADEM. MOGAE refers to an autoimmune encephalitis manifestation of MOGAD, contrasting with more commonly seen phenotypes like optic neuritis and myelitis in MOGAD. MOGAE presents with multiple symptoms, including encephalopathy, seizures, focal neurological signs, and seizures [7].
There have been several reports of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2)-triggered MOGAD and autoimmune encephalitis cases after coronavirus disease 2019 (COVID-19), but there are no cases of MOGAE negative for relevant brain MRI findings. Here, we report a case of MRI-negative MOG encephalitis after COVID-19 in a patient with a previous history of encephalitis.
Case report
This case report was approved by the Institutional Review Board (IRB) of the Seoul National University Hospital (No. H-2211-142-1381), and the requirement to collect informed consent from the patient was waived by the IRB.
A 50-old man with a history of well-controlled localization-related epilepsy was presented to our hospital because of visual and auditory hallucinations. The patient had a history of 2 previous episodes of encephalitis. Forty years ago, he had experienced a generalized tonic-clonic seizure and was diagnosed with encephalitis after cerebrospinal fluid (CSF) tapping. Fifteen years ago, he showed headache, sensory aphasia, and seizure and was presumed to have viral encephalitis with CSF pleocytosis (white blood cell count, 126/mm3; lymphodominant); he ultimately fully recovered with antiviral agent treatment. Six days prior to the current admission, he experienced a fever and mild headache without other respiratory symptoms, and nasopharyngeal rapid antigen and nasopharyngeal polymerase chain reaction (PCR) tests were positive for SARS-CoV-2 infection. Eight days after the onset of COVID-19 symptoms, he had generalized seizures of about 3 minutes in duration and left-side paresthesia. Thirteen days after COVID-19 symptom onset, the patient visited the emergency room for recurrent left paresthesia and auditory hallucinations. No more seizures were documented, and he was prescribed antiseizure medication (levetiracetam, 1,500 mg/day) and discharged. The evening after discharge from the emergency room, while watching TV, he heard the same song over and over again and complained that the screen looked strange. He also complained of auditory hallucinations, such as machine sounds and cheering songs, visual hallucinations of someone else performing cardiopulmonary resuscitation on him, and feeling that he was about to die. The patient also complained of decreased sensation in the left face and arm. He returned to the emergency room with repeated focal status epilepticus with impaired consciousness showing unresponsiveness, automatism, and incoherent speech over 10 minutes. The timeline of the clinical course and treatment of the patient is summarized in Figure 1.
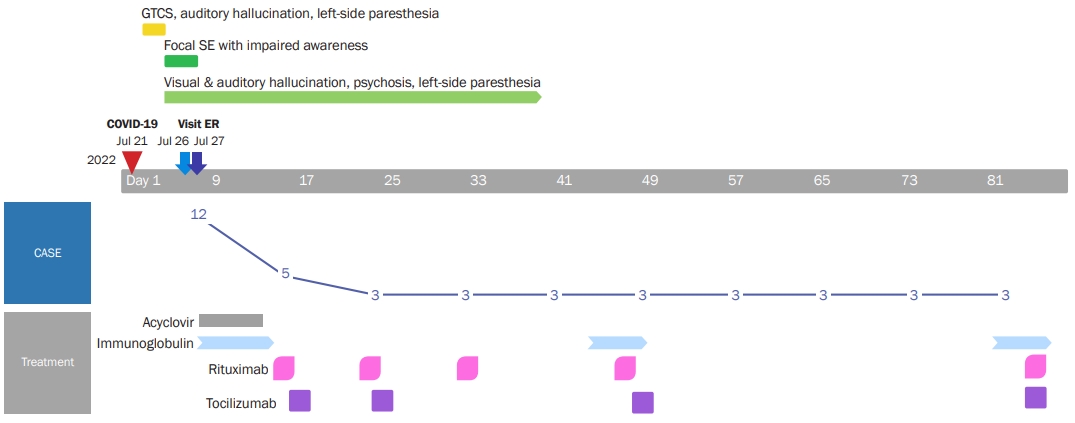
Timeline of clinical course and treatment of the patient
GTCS, generalized tonic-clonic seizure; SE, status epilepticus; COVID-19, coronavirus disease 2019; ER, emergency room; CASE, Clinical Assessment Scale for Autoimmune Encephalitis.
No specific findings were recorded following the cranial nerve assessment during the neurological examination performed when the patient’s cooperation was possible, and the neurologic deficit was not prominent. The patient showed repetitive unresponsiveness and disorganized speech. Brain MRI showed a few tiny nonspecific T2 hyperintensities in bilateral cerebral white matter and no abnormal focal enhancing lesions (Figure 2A). CSF study showed pleocytosis (white blood cell count, 33/mm3 [lymphodominant]; red blood cell count, 280/mm3; protein concentration, 41 mg/dL; CSF glucose concentration, 59 mg/dL [serum concentration, 101 mg/dL]), and viral PCR (herpes simplex virus 1, herpes simplex virus 2, Epstein-Barr virus, cytomegalovirus, varicella-zoster virus, enterovirus, respiratory virus, herpes virus 6, herpes virus 8, and ohn Cunningham virus) tests were all negative. The CSF SARS-CoV PCR result was also negative. Tests for autoimmune synaptic antibodies (NMDAR, LGI1, CASPR2, AMPAR, DPPX, GABAʙR) and paraneoplastic antibodies (Hu, Yo, Ri, amphiphysin, Ma2/Ta, CA2, recoverin, SOX2) were negative. The aquaporin-4 antibody result was also negative, but the MOG antibody result was positive according to flow cytometry (ratio of positive cells) was positive (0.692; positive, >0.254). The mean fluorescence intensity of the fluorescein channel was also positive (9.12; positive, >3.65). The CSF oligoclonal band was positive, with the paired sera showing a normal polyclonal response. Electroencephalography (EEG) revealed a right-dominant moderate amount of generalized theta to delta slow waves with occasional lateralized semi-rhythmic delta activities (Figure 3A). Levetiracetam, which may show psychiatric side effects, was changed to lacosamide (200 mg/day). The patient could be diagnosed with possible autoimmune encephalitis with focal status epilepticus with impaired awareness following a preceding infection and abnormal findings on EEG.
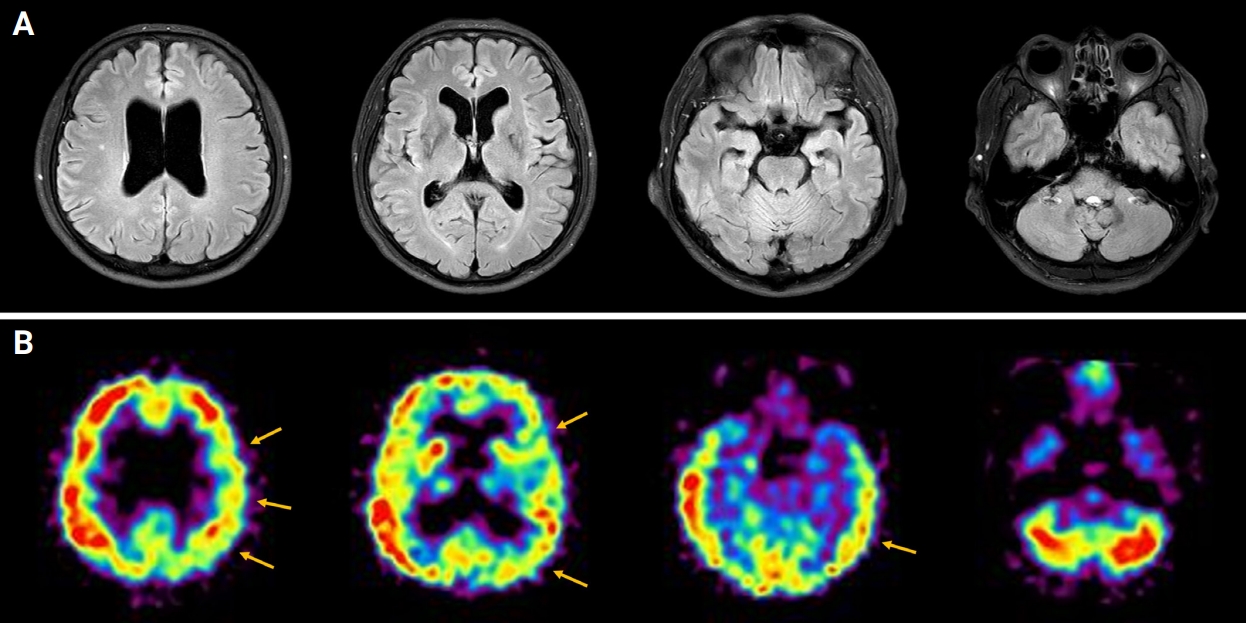
Brain MRI and brain SPECT findings
(A) Brain MRI showed a few nonspecific hyperintensities in bilateral cerebral white matter on a T2 fluid-attenuated inversion recovery image. (B) Brain SPECT showed relative and mild cerebral perfusion asymmetry at the left fronto-parieto-temporal cortex (yellow arrows).
MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography.
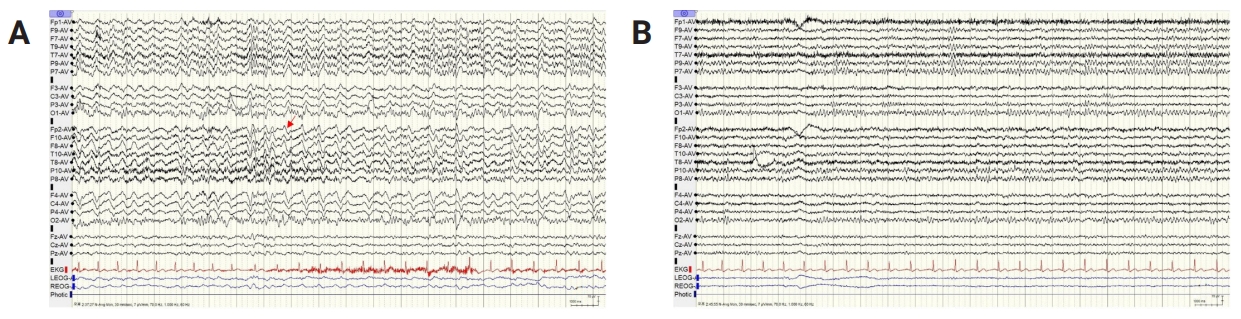
EEG findings
(A) The initial EEG demonstrated a right-dominant moderate amount of generalized theta to delta slow waves with occasional lateralized semi-rhythmic delta activities (red arrow). (B) A follow-up EEG showed a well-regulated 9-Hz posterior dominant rhythm.
EEG, electroencephalography.
Since infectious encephalitis was not excluded, high-dose corticosteroid was not considered as a first-line treatment. Intravenous acyclovir (discontinued after negative viral PCR results) and intravenous immunoglobulin treatment (0.4 g/kg for 5 days) were initiated. The patient’s psychotic symptoms remained, and a positive MOG antibody result was confirmed. The patient was diagnosed with MOGAE and started on rituximab (375 mg/m2 at regular intervals; the first 4 doses were administered weekly, with monthly dosing thereafter) on hospital day 9 and tocilizumab (4 mg/kg for 1 day; 2 doses given every 2 weeks, then doses given once a month) on hospital day 10. The patient’s aggressive and violent behavior led to us considering admission to a psychiatric ward. In the acute period of severe psychotic symptoms, risperidone 4 mg/day was administered, and haloperidol 5 mg together with lorazepam 4 mg were given when needed during the acute period of severe psychotic symptoms. Risperidone 1 mg/day was used as maintenance treatment after the acute period. The patient was stabilized with antipsychotic treatment and discharged on the 22nd day of hospitalization. Most of his symptoms improved 2 weeks after discharge, but intermittent auditory hallucination misperceptions of TV sounds and autotopagnosia of the left hemibody remained. Brain single-photon emission computed tomography imaging on the 38th day after hospitalization showed relative and mild cerebral perfusion asymmetry at the left fronto-parieto-temporal cortex (Figure 2B). A follow-up EEG recorded on the 37th day after hospitalization improved with a small amount of generalized theta slowing and normal reactive posterior dominant rhythm (Figure 3B).
Discussion
We report the case of a patient who presented with focal status epilepticus with impaired awareness and psychiatric symptoms, including auditory hallucinations, and CSF pleocytosis after SARS-CoV-2 infection. Also, the MOG antibody test result was positive. The patient was diagnosed with MOGAE based on clinical symptoms and electrographic and serologic findings. In this case, the cause of the previously remote pair of episodes of encephalitis was unclear, but it is thought that MOG antibodies were produced due to his SARS-CoV-2 infection.
There are many reports about the mechanism of autoimmune diseases and neurological diseases related to coronavirus infections in the COVID-19 pandemic era. There was a report that SARS-CoV-2 IgG antibody levels were increased in MOGAD patients compared to MOG-antibody-seronegative age- and time-matched controls in the pandemic period, so SARS-CoV-2 seems to be able to trigger MOGAD [8]. Several case reports and case series have reported about SARS-CoV-2–positive patients presenting with MOGAD. According to case reports of patients diagnosed with MOGAD after SARS-CoV-2 infection, various manifestations, such as bilateral optic neuritis, encephalomyelitis, ADEM, and MOGAD relapse, were observed, and MOGAD is thought to be caused by a postinfection immune-mediated reaction mechanism [9,10]. Previously published case reports and series of MOGAD following COVID-19 are summarized in Table 1 [10-20]. Woodhall et al. [11] reported a case of relapsing MOGAD after COVID-19 where a female patient with a history of MOGAD was confirmed to have a MOG-IgG1 antibody titer of below the 1:200 cut-off; 6 days after COVID-19 diagnosis, she experienced ocular pain, and decreased visual acuity. MOG-IgG1 seroreversion was confirmed at MOG-antibody titer to 1:800. In this case, the cause of his previous two episodes of encephalitis was unclear, but it is thought that MOG antibodies were produced due to our patient’s SARS-CoV-2 infection.
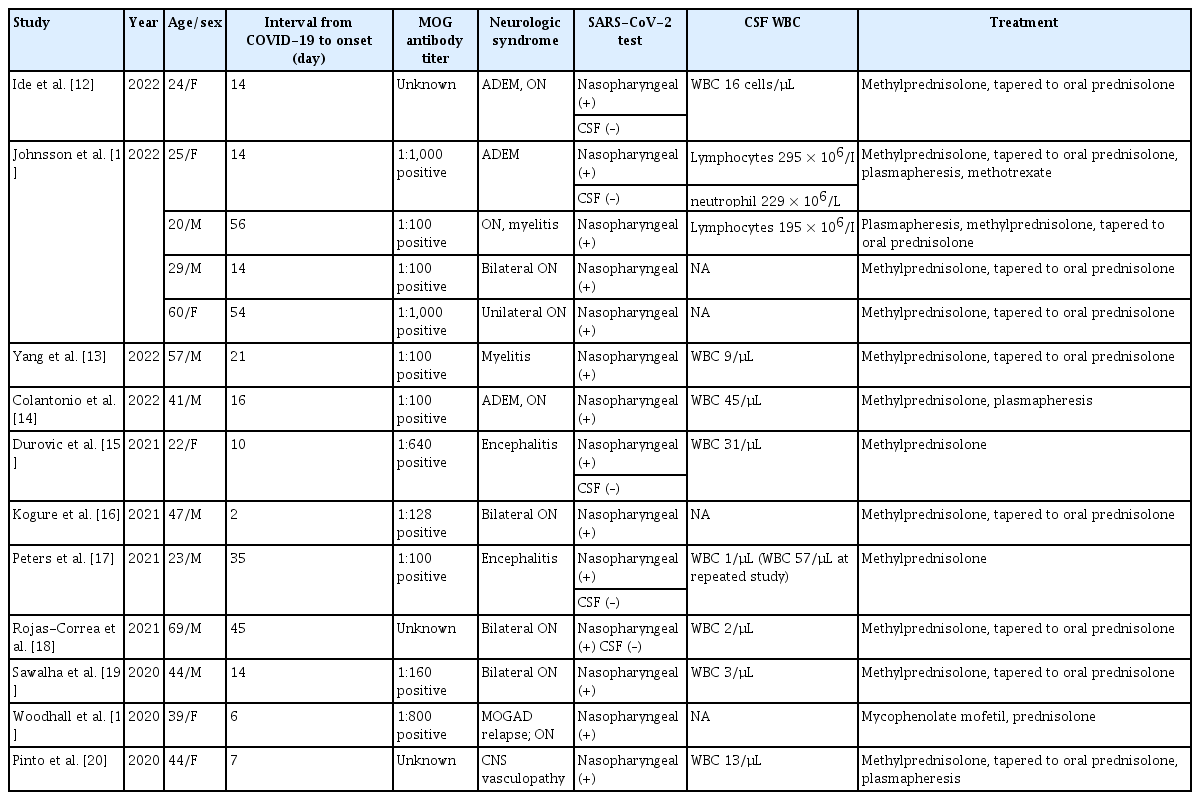
Previously published case reports and series of MOG antibody-associated disease following SARS-CoV-2 infection
This case is also significant in that the patient had normal brain MRI MOGAE findings. There are reports of spine MRI-negative cases in MOGAD that occurred after COVID-19, but there has been no report of brain MRI-negative cases like this case. As mentioned above, considering the rate of MOGAD cases in which brain MRI and spine MRI abnormalities were reported, on the contrary, about half of patients may not report any abnormal findings on MRI [4]. Among recently reported cases of image-negative myelitis, Macaron and Ontaneda [21] reported a 57-year-old man who developed urinary retention, symmetric paraparesis, and hypoesthesia in both lower extremities after an upper respiratory infection 1 week prior. Separately, Park et al. [22] reported the case of an 18-year-old man who presented with hypoesthesia and weakness in both extremities. In both of these patients, no other cause of myelitis was identified except for MOG antibodies as a result of live cell-based fluorescence-activated cell sorting assay. Sechi et al. [23] reported a retrospective study of MOG-IgG–associated myelitis, which was performed within 6 weeks of onset at a single center from 2000 to 2019; notably, the initial spinal cord MRI result was negative in 10% of patients. Although the cause of the discrepancy between these clinical symptoms and MRI findings is unclear, there is an explanation that MRI cannot sufficiently detect MOG-IgG–associated inflammation, such as image timing, MOG-IgG–related spinal meningitis, and functional disturbance due to MOG-IgG [23]. The patient in this report was treated with acyclovir for CSF pleocytosis, and IVIG treatment was started early considering the possible autoimmune encephalitis. High-dose corticosteroid was not administered because an infectious cause had not been completely ruled out. To address the remaining clinical symptoms, maintenance treatment was performed using rituximab and tocilizumab. Autoimmune encephalitis, including MOG encephalitis occurring after COVID-19, usually shows a favorable response to immunotherapy [12,13]. Also, in this case, focal status epilepticus, uncontrolled seizures, and severe psychosis were alleviated early with intensive immunotherapy. The diagnosis and treatment of MOGAE have not yet been established. Referring to the results of an international survey on MOG antibody treatment published in 2020, high-dose corticosteroids are administered for an acute attack, and plasma exchange or intravenous immunoglobulin is administered if the treatment response is insufficient. Azathioprine, mycophenolate mofetil, and rituximab are generally employed as first-line maintenance therapeutics in these cases [24]. Tocilizumab, an interleukin-6 receptor blockade, was not favored as a first-line treatment, but interleukin-6 plays an important role in experimental autoimmune encephalitis, a murine model of MOGAD, and CSF interleukin-6 levels increase in the acute period of MOGAD patients [25,26]. There was a report that rituximab-refractory MOGAD induces remission [27]. Tocilizumab may also be an effective treatment option for patients with MOGAD.
Our case patient initially had severe psychotic symptoms, including aggression and auditory hallucinations, to the extent that admission to a psychiatric hospital and isolation ward was considered, but his symptoms gradually improved with antipsychotics, rituximab, and tocilizumab treatment. Among the CASE (Clinical Assessment Scale for Autoimmune Encephalitis) score items used in the clinical symptoms assessment scale of patients with autoimmune encephalitis, psychiatric symptoms (0, none; 1, mild; 2, moderate; and 3, severe) improved from 3 points (needs continuous care or admission because of the psychiatric symptom) to 2 points (needs medical intervention because the symptom interferes with daily activities). When the clinical characteristics of MOGAE were investigated in adult patients, memory disturbance was the most common symptom (82.5%), followed by seizures (62.5%), psychiatric symptoms (60.0%), language problems (67.5%), consciousness changes (55%), dyskinesia, dystonia, and ataxia [7,28].
In summary, we report the case of a 50-year-old man with MRI-negative MOGAE after COVID-19 and note that COVID-19 could trigger autoimmune encephalitis conditions like MOGAE. MOG antibody testing can be considered even if the MRI is negative in suspected post–SARS-CoV-2 autoimmune encephalitis.
Notes
Conflicts of Interest
Jangsup Moon has been an associate editor of encephalitis since October 2020. Sang Kun Lee and Kon Chu have been the editorial boards of encephalitis since October 2020. They were not involved in the review process of this original article. No other potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Chu K; Formal analysis, Project administration: Moon J; Visualization: Lee Y, Lee HS, Kim YM, Lee S, Park HY; Investigation: Kim Y, Lee S; Software: Ahn SJ; Supervision: Ahn SJ, Lee SK, Chu K; Validation: Lee HS, Park H, Lee SK; Writing–original draft: Lee Y, Moon J; Writing–review & editing: Ahn SJ, Lee SK, Chu K.
